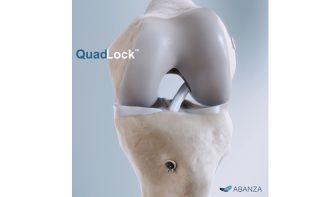
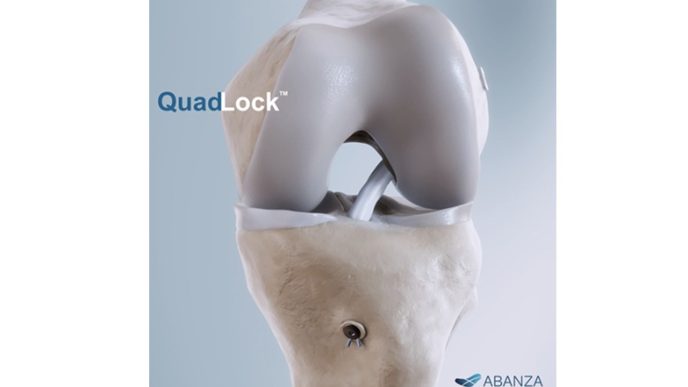
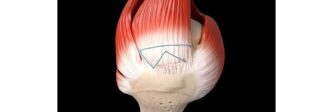
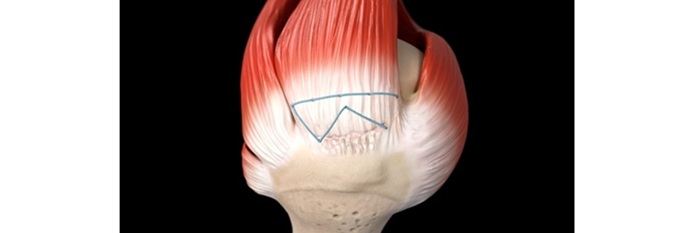
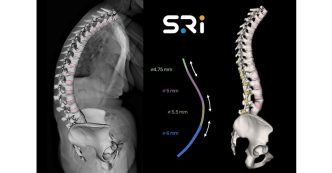
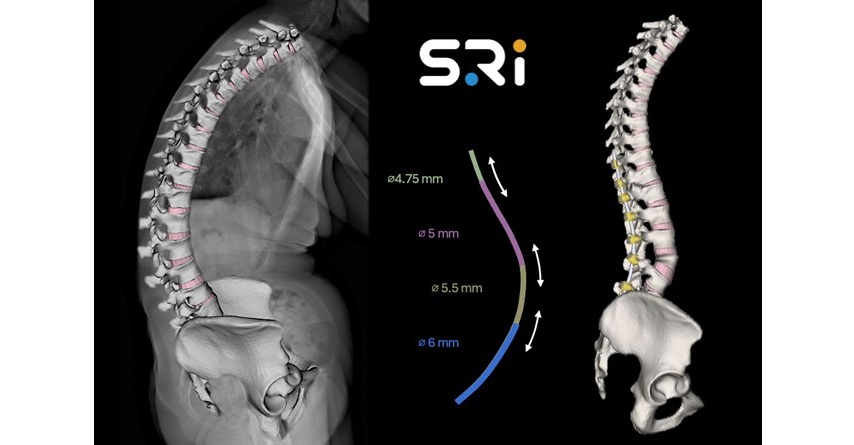
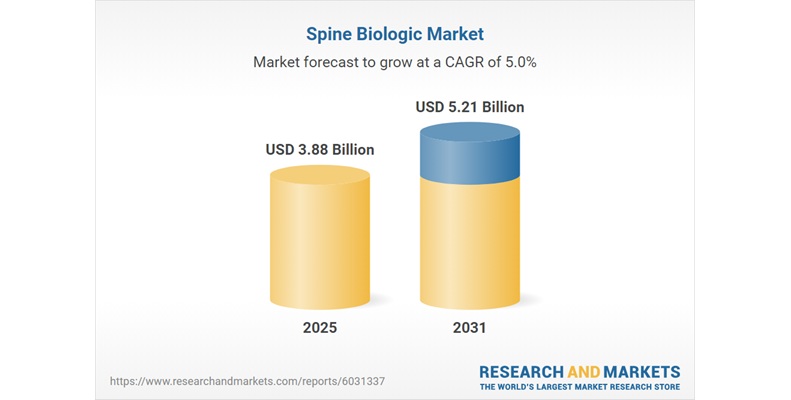
ABANZA Secures FDA 510(k) Clearance for QuadLock™ Fixation System for ACL Reconstruction
ABANZA’s QuadLock™ delivers market-leading fixation strength with over a 500% reduction in cyclic displacement compared to conventional fixation methods. ORLANDO, Fla., Jan. 27,…