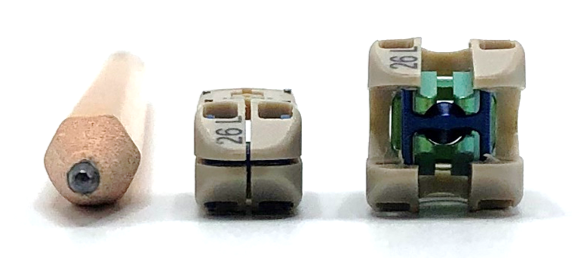
New 7mm insertion profile, combined with company’s proprietary Adaptive Geometry™ technology, are designed to respect patients’ neural, vascular, bony and soft tissue anatomy both during and after insertion to promote long-term stability
PALM BEACH GARDENS, Fla., Aug. 17, 2021 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced the launch of FlareHawk7, a multidirectionally expandable lumbar fusion device that is inserted at an ultra-low profile of 7mm to help minimize neural retraction before expanding in both the cranial-caudal and medial-lateral planes to provide sagittal and coronal correction, foraminal height restoration, and the stability to promote fusion.
The FlareHawk7 platform also features a suite of instruments designed to facilitate surgeons’ technique preferences. For endoscopic TLIF procedures, surgeons can leverage access with instruments to allow direct visualization of disc preparation and implant delivery. For MIS procedures, surgeons are provided access to the disc space through Kambin’s triangle while also preserving the patient’s normal anatomy. And in a “mini-open” procedure, the need for neural retraction can be minimized thanks to the smaller insertion footprint that is similar in profile to a No. 2 pencil.
“FlareHawk7 enables me to do fully endoscopic interbody fusion as outpatient surgery, with the best endplate prep and great clinical results,” said Jian Shen, M.D., PhD, a world-renowned leader in endoscopic spine surgery.
FlareHawk7 permits concurrent expansion in height and width to restore disc height without sacrificing stability. It enters the disc space with a compact 7mm x 7mm profile and then expands up to 11mm x 12mm—a 57% increase in width and 72% increase in height—with 0° and 6° lordosis options. This provides a larger surface contact area to distribute load with the goal of reducing subsidence in weak, osteoporotic bone. The implant’s open architecture design also allows significant graft delivery through the implant and into the surrounding disc space.
“FlareHawk7 is a game changer,” said Jason Huffman, M.D., a recognized leader in minimally invasive spinal fusion and Director of the Huffman Clinic in Napa, Calif. “It enables me to quickly and effectively perform a thorough discectomy and interbody fusion with a kick-ass, multiplanar-expandable, structured cage while utilizing a minimally invasive, percutaneous approach through Kambin’s triangle.”
Dr. Paul Houle, M.D., board-certified neurosurgeon with Cape Cod Healthcare Neurosurgery in Hyannis, Mass. agrees. “Historically, my surgical options were greatly limited when patients presented with scarring,” Dr. Houle said. “However, FlareHawk’s narrow insertion profile means I can treat these patients from a single position posteriorly – something not offered by other products currently on the market.”
The first-of-its-kind FlareHawk® family of implants utilizes a PEEK shell and internal shim, providing a small insertion profile to minimize neural retraction during insertion and then allowing expansion in height, width and lordosis, designed to reduce subsidence, restore foraminal height and re-establish sagittal balance. Peer-reviewed studies have suggested FlareHawk’s Adaptive Geometry to be a potential contributor to the implant’s positive fusion rates, as well as to the absence of observed subsidence1-4, a concern with monolithic cages that present a mismatch in the modulus of elasticity between the implant and the bone. To date, over 10,500 FlareHawk cages have been implanted in more than 9,000 patients.
“No other implant represents our philosophies of Adaptive Geometry and Access Without Compromise™ as well as FlareHawk7 with its ultra-low 7mm insertion profile expanding to 11mm, a full 2mm wider than the average 9mm TLIF implant. This allows for less neural retraction yet more footprint than current devices,” said Chris Walsh, CEO of Accelus. “We refuse to be dogmatic in our approach, as FlareHawk7 enables endoscopic, percutaneous, mini-open, and two-implant open PLIF procedures—all benefiting from Accelus’s Access without Compromise promise.”
About FlareHawk Expandable Lumbar Interbody Fusion System
The FlareHawk Interbody Fusion System is indicated for spinal intervertebral body fusion with autogenous bone graft and/or allogeneic bone graft composed of cancellous and/or corticocancellous bone in skeletally mature individuals with degenerative disc disease (DDD) at one or two contiguous levels from L2 to S1, following discectomy. DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have at least six (6) months of non-operative treatment. Additionally, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). FlareHawk system spacers are intended to be used with supplemental fixation instrumentation, which has been cleared for use in the lumbar spine.
About Accelus
Accelus is committed to accelerating minimally invasive spine surgery through its procedure-enabling technology with broad accessibility to previously underserved markets. Founded in 2021 through the combination of Integrity Implants and Fusion Robotics, the company is focused on providing its proprietary Adaptive Geometry technology with pragmatic and economical navigation and robotic solutions with broad clinical use in spine surgery.
- Cheng BC, Swink I, Yusufbekov R, et al. Current Concepts of Contemporary Expandable Lumbar Interbody Fusion Cage Designs, Part 1: An Editorial on Their Biomechanical Characteristics. International Journal of Spine Surgery. https://www.ijssurgery.com/content/14/s3/S63. Published December 1, 2020. Accessed July 27, 2021.
- Cheng BC, Swink I, Yusufbekov R, Birgelen Michele, Ferrara L, Coric D. Current Concepts of Contemporary Expandable Lumbar Interbody Fusion Cage Designs, Part 2: Feasibility Assessment of an Endplate Conforming Bidirectional Expandable Interbody Cage. International Journal of Spine Surgery. https://www.ijssurgery.com/content/14/s3/S68. Published December 1, 2020. Accessed July 27, 2021.
- Coric D, Roybal RR, Grubb M, et al. Bidirectional Expandable Technology for Transforaminal or Posterior Lumbar Interbody Fusion: A Retrospective Analysis of Safety and Performance. International Journal of Spine Surgery. https://www.ijssurgery.com/content/14/s3/S22. Published December 1, 2020. Accessed July 27, 2021.
- Tan LA, Rivera J, Tan XA, Le VP, Khoo LT, Berven SH. Clinical and Radiographic Outcomes After Minimally Invasive Transforaminal Lumbar Interbody Fusion-Early Experience Using a Biplanar Expandable Cage for Lumbar Spondylolisthesis. International Journal of Spine Surgery. https://www.ijssurgery.com/content/14/s3/S39. Published December 1, 2020. Accessed July 27, 2021.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/06410131-d598-4c8c-a4fb-ef00c23db9ce
Media Contact: Brandy Craig
305-676-1679
bcraig@accelusinc.com
Source: Accelus