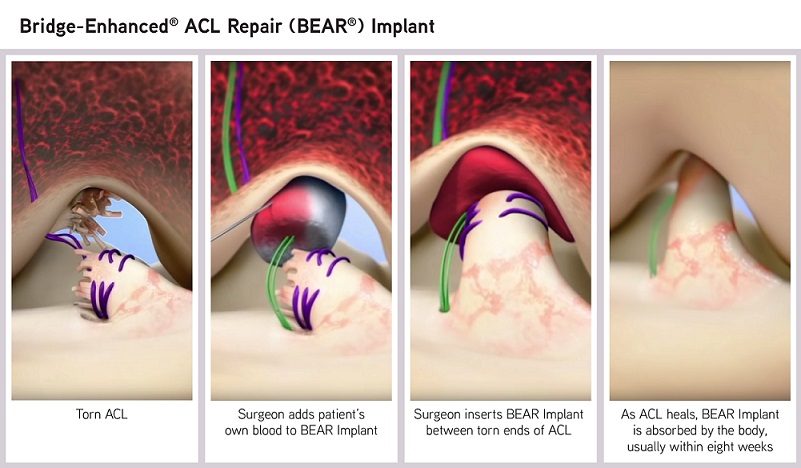
World’s First Artificial Meniscus Prosthesis is Now Available in Switzerland
September 6, 2021
MEMPHIS, Tenn.–(BUSINESS WIRE)–Active Implants LLC, a company that develops orthopedic implant solutions, today announced that it has reached an exclusive agreement with Geistlich Pharma AG to be the exclusive Swiss distributor of the company’s NUsurface® Meniscus Prothesis. The world’s first “artificial meniscus” is now available in Belgium, Germany, Israel, Italy, Switzerland and the UK.
The relationship between Active Implants and Geistlich Pharma began in 2019 with the launch of the MEFISTO project. The international effort under the prestigious EU Horizon2020 program is designed to develop interventions focused on joint preservation and delaying or preventing joint-sacrificing procedures such as knee replacement, thus reducing the social burden, associated costs and high levels of morbidity resulting from osteoarthritis (OA).
“With Geistlich Pharma’s focus on knee preservation through the regeneration of bone, cartilage and tissue, we feel confident that our two organizations are aligned in our core values,” said Ted Davis, president and CEO of Active Implants. “We look forward to expanding access to the NUsurface through their world-class medical education, training and support.”
The NUsurface is the first artificial meniscus to be marketed in Europe and is currently under review by the U.S. Food and Drug Administration (FDA). The medial meniscus replacement mimics the function of the natural meniscus and treats pain by redistributing loads transmitted across the knee joint. The NUsurface offers patients suffering from meniscus-deficient knee pain a safe and effective surgical alternative to knee replacement, an invasive procedure that is considered the current standard of care for patients unable to get relief from bracing/pain medications or arthroscopic surgery.
About the NUsurface® Meniscus Prosthesis
The NUsurface® Meniscus Prothesis is an artificial meniscus made from medical grade polymer. As the result of its unique materials, composite structure and design, NUsurface does not require fixation to bone or soft tissues. NUsurface mimics the function of the natural meniscus by acting as a shock absorber to help distribute weight transmitted across the knee joint, reducing pain and improving physical function. By limiting stress and protecting femoral articular cartilage from further damage, the NUsurface may be chondroprotective. It is currently marketed in Belgium, Germany, Israel, Italy, Switzerland and the UK.
Results from two U.S. prospective, concurrent, clinical trials presented at the recent American Orthopedic Medicine Society-Arthroscopy Association of North America Combined 2021 Annual Meeting demonstrated that the NUsurface provided superior relief from post meniscus surgery knee pain when compared to treatment with non-surgical care alone.
About Active Implants, LLC
Active Implants, LLC, develops orthopedic implant solutions that complement the natural biomechanics of the musculoskeletal system, allowing patients to maintain or return to an active lifestyle. Active Implants is privately held with headquarters in Memphis, Tennessee. European offices are in Haarlem, The Netherlands, with R&D facilities in Netanya, Israel. For more information, visit www.activeimplants.eu. Follow the company on Twitter, LinkedIn and Facebook.
About Geistlich Pharma AG
Geistlich Pharma AG is a Swiss company, owned by the family Geistlich with its headquarters in Central Switzerland in Wolhusen and in Root. The company is part of the Geistlich Holding. Since the business was established in 1851, Geistlich has developed its expertise in the processing of bone and tissue.
Geistlich Pharma specializes in the regeneration of bone, cartilage and tissue. Geistlich’s regenerative medical devices aim to improve patients’ quality of life. More than 700 employees worldwide work for Geistlich in the area of regenerative medicine. With its twelve affiliates and 60 distribution partners, Geistlich’s medical devices and medicinal products reach around 90 markets worldwide. The twelve affiliates cover the UK, Germany, Italy, France, China, Brazil, South Korea, North America, Australia, New Zealand, India and Japan.
Geistlich Surgery is a business unit of Geistlich Pharma AG.
About MEFISTO
Launched 1 April 2019, MEFISTO (Meniscal functionalised scaffold to prevent knee Osteoarthritis onset after meniscectomy) is a joint effort of 13 partners from academia, hospitals and industry from eight different European countries to develop novel solutions to treat meniscus loss as a strategy for preventing the onset of an epidemic of post-meniscectomy knee osteoarthritis (OA) in Europe. MEFISTO receives funding from the European Union’s Horizon 2020 research and innovation programme. The project will run until 30 November 2023. MEFISTO is coordinated by Geistlich Biomaterials Vertriebsgesellschaft mbH BIOMATERIALS Germany.
Contacts
Joni Ramirez
Merryman Communications
joni@merrymancommunications.com
323.532.0746