September 7, 2021
KARLSRUHE, Germany–(BUSINESS WIRE)–German-based joimax®, the market leader in technologies and training methods for full-endoscopic and minimally invasive spinal surgery, is excited to announce that it has received FDA clearance to market the EndoLIF® Delta-Cage and EndoLIF® DoubleWedge-Cage in the United States. Delta-Cages and DoubleWedge-Cages are intended for intervertebral body fusion procedures for various diseases of the lumbar spine, such as degenerative disc disease.
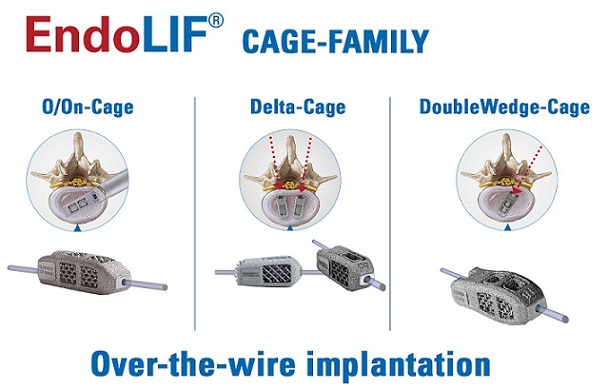
Now in its 20th anniversary year, joimax® sets another milestone by providing a family of 3D printed titanium cages. The complete EndoLIF® product portfolio consists of the Delta-Cage, the DoubleWedge-Cage, and the O/On-Cage. All three models are designed with special features:
- 3D printed titanium for excellent bone ingrowth, fillable with bone or bone substitute material, pre- or post-insertion
- Anatomical shape and lordotic correction options for OLIF, TLIF, and PLIF procedures
- “Over-the-wire” implantation for optimal targeted positioning in the intervertebral disc space
- Endoscopic control of all surgical steps due to the EndoLIF® platform technology
“It is impressive how well these cages ensure primary stability, especially the DoubleWedge-Cage,” says Dr. Ralf Wagner, head surgeon and founder of the Ligamenta Spine Centre in Frankfurt.
Many other experts including, Dr. Florian Zentz, head surgeon of the spine center in Tutzing (Munich), Dr. Ricardo Casal Grau, orthospine surgeon from Madrid, and Dr. J. N. Alastair Gibson, spinal surgeon from Edinburgh, supported the development of the joimax® cages.
“With FDA clearance of two of our cages, our EndoLIF® platform gets more complete; the next major step in endoscopic spinal surgery is endoscopically-assisted fusion,” says joimax® Founder and CEO Wolfgang Ries. “More and more centers are proving the efficacy of these procedures in studies, demonstrating once again the major benefits of endoscopy in fusion. So far, our cages are well established in Europe and in selected centers in Asia, and now we are entering the USA, the biggest market for spinal fusion”.
About joimax®
Founded in Karlsruhe, Germany in 2001, joimax® is the leading developer and marketer of complete systems for full-endoscopic and minimally invasive spinal surgery. With the Endoscopic Surgical Systems TESSYS® (transforaminal), iLESSYS® (interlaminar) and CESSYS® (cervical) for decompression procedures, MultiZYTE® for facet and sacroiliac joint pain treatment, EndoLIF® and Percusys® for minimally-invasive endoscopically assisted stabilizations, established systems are provided, addressing a whole range of indications. In procedures for herniated disc, stenosis, pain therapy or spinal stabilization treatment, surgeons utilize joimax® technologies to operate through small incisions under local or full anesthesia, via tissue and muscle-sparing corridors, and through natural openings in the spinal canal, e.g. the intervertebral foramen, the so-called “Kambin triangle”.
Contacts
Press Contact Germany
joimax® GmbH
Nicole Read
Nicole.Read@joimax.com
+49-721-25514-0