November 8, 2021
CARLSBAD, Calif.–(BUSINESS WIRE)–Spinal Elements, a spine technology company, today announced that the Lucent 3D Lumbar Interbody System has been successfully used in its first clinical cases with Dr. Thomas Noh, Neurosurgeon at Hawaii Pacific Health and Assistant Clinical Professor at the John A. Burns School of Medicine (JABSOM) at the University of Hawaii and Dr. Douglas H. Musser, D.O. at Youngstown Orthopedics Associates in Canfield, Ohio.
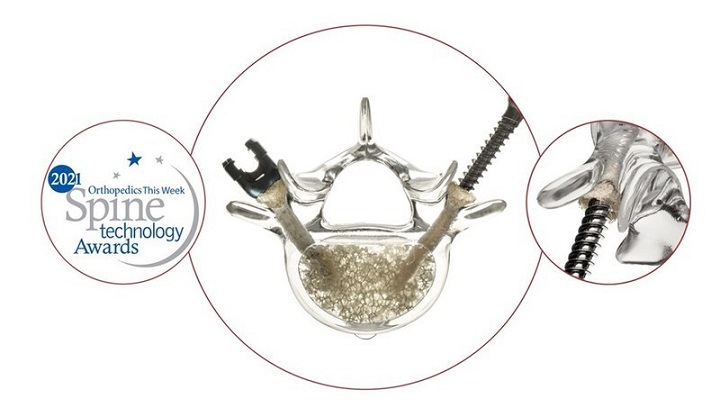
The Lucent 3D Lumbar Interbody System features a functionally unique multi-component device manufactured in a 3D printing sequence. The resulting Lucent 3D implant is comprised of a strut-and-lattice structure with a bone graft chamber access lid designed to allow the surgeon to deliver a large amount of tightly packed graft inside the interbody structure, maximizing the amount of graft material available for fusion. Furthermore, when the access lid to the graft chamber is closed, the lid is designed to maximize surface area in contact with the vertebral endplates. Provided in both straight and curved designs, the interbody device is designed to be placed through an open or MIS posterior approach.
Dr. Noh commented, “The Lucent 3D Interbody is a novel and useful surgical option in my practice. The curved implant was easy to insert and helped to maintain lordosis. Knowing that there is tightly packed graft inside the cage provides additional confidence that a successful fusion will occur.”
Jason Blain, CEO of Spinal Elements, stated, “We are thrilled to share this technology with the surgical community. Lucent 3D’s novel design is meant to address subsidence and the amount of bone graft material available for fusion – two clinical challenges surgeons note exist with other 3D-printed interbody devices. We are pleased to offer additional innovative titanium interbody devices to our expansive and best-in-class product portfolio, adding to our long tradition of innovation in material science, novel designs, and leadership. We truly can answer any need for posterior lumbar fusion and continue to build on our procedural solutions.”
After finishing his first several Lucent 3D cases, Dr. Musser commented, “The design and structure of the Lucent 3D cage have provided a biologic containment area that has enabled me to place more graft material than traditional PLIF and TLIF cages. The Lucent 3D cage also has excellent visualization on both A/P and Lateral views when using fluoroscopy.”
Spinal Elements announced FDA clearance of the Lucent 3D system in April of this year and expects to expand on Lucent 3D’s graft containment concept in other clinical applications.
About Spinal Elements
Spinal Elements is a Carlsbad, California based medical device company focused on the design, development, and commercialization of a comprehensive portfolio of systems, products, and technologies for spine surgery procedures. A leading designer, developer, manufacturer, and marketer of innovative medical devices used in spinal surgical procedures, Spinal Elements combines leading medical device technologies, biologics, and instrumentation to create positive surgical outcomes that exceed surgeon and patient expectations. Spinal Elements has built a reputation delivering innovative and differentiated technologies that enable fundamental shifts in solutions for spine surgery. The company markets a complete portfolio of advanced spinal implant technologies.
For more information, please visit www.spinalelements.com.
Contacts
For interviews or more information, contact:
Laura Charlton (formerly Johnson) for Spinal Elements
laurajohnsonpr@yahoo.com (760) 450-7749 cell