CHATTANOOGA, Tenn., June 29, 2022 /PRNewswire/ — 3Spine, Inc., a medical device company developing total joint replacement for the lumbar spine, today announced completion of the first series of US surgeries in the BalancedBack Total Joint Replacement Investigational Device Exemption (IDE) pivotal clinical trial. All patients enrolled in the IDE will receive the MOTUS device and will be propensity matched against patients prospectively enrolled in 3Spine’s real-world evidence (RWE) fusion study, using an adaptive statistical design. 3Spine’s RWE study has been enrolling in the US since May 2021.
Benjamin Geddes, MD performed the surgeries at the Center for Sports Medicine and Orthopaedics Surgery Center in Chattanooga, TN, assisted by Alex Sielatycki, MD of Steamboat Orthopaedic and Spine Institute and UC Health Yampa Valley Medical Center in Steamboat Springs, CO. “Alex and I are humbled to have the honor of performing the first lumbar total joint replacements in the US,” said Dr. Geddes. “The procedures went very well. Each patient was up and walking within 2 hours after surgery, using the early mobilization protocols for hip and knee arthroplasty patients. This is an encouraging start and we look forward to continuing enrollment.”
Jeff Goldstein, MD, national principal investigator (PI) for the IDE said, “On behalf of our study PIs around the country, I’d like to congratulate Dr. Geddes on being the first site to complete enrollment of the RWE study, making him eligible to begin enrollment of the IDE. This is historic work.” Dom Coric, MD, national PI for the RWE study continued, “Our strategy to first collect real-world evidence on a variety of posterior lumbar fusions is working well, providing an important baseline at each site and allowing for a staged start to the IDE across centers. I echo Jeff in congratulating Alex and Ben on their achievement.”
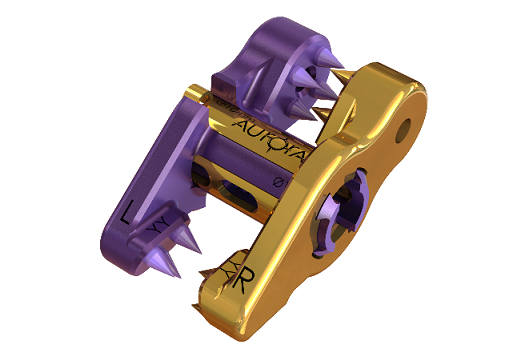
3Spine’s MOTUS device, the implant used in the BalancedBack Total Joint Replacement procedure, is a ‘first of kind’ technology replacing the function of the disc and facet joints through a posterior approach. The procedure is intended to broadly address leg pain, back pain, and spinal instability, while correcting posture and restoring freedom of movement through reconstruction of the functional spinal unit. 3Spine is seeking single-level indications from L1-S1 in patients suffering from lumbar degeneration with or without foraminal or recess spinal stenosis with no more than a grade 1 spondylolisthesis at the involved level. Additional details are available at ClinicalTrials.gov.
About 3Spine
3Spine is a new kind of healthcare company founded to integrate the development, clinical research, and delivery of low back total joint replacement. 3Spine is headquartered in Chattanooga, TN, with research and development facilities in the Greater Boston area and clinical operations in the Cayman Islands. www.3spine.com
Media Contact
Christopher Chafin, SweeneyVesty New York
chris.chafin@sweeneyvesty.com
Tel 718 530 4135
SOURCE 3Spine