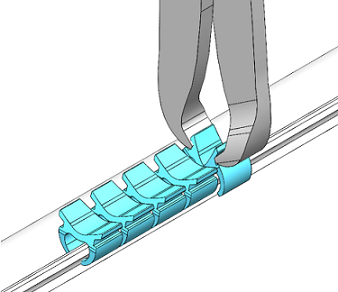
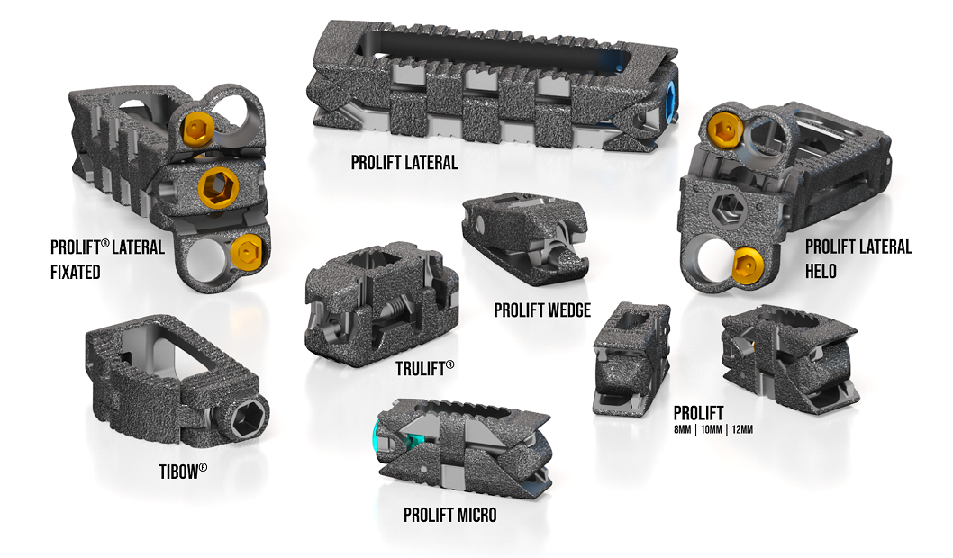
Salt Lake City, Aug. 30, 2022 /OrthoSpineNews/ – Innovasis®, the leader in Bioactive spine applications, today announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its HAcancellous™ PEEK-C, a Porous Cervical IBF System. HAcancellous™ PEEK-C is made from Invibio® PEEK-OPTIMA® HA Enhanced1 and includes Pore Matrix™ Technology2. Hydroxyapatite (HA) is fully integrated throughout the implant, including the porous layers. The HAcancellous PEEK-C Implant is made from PEEK which has a modulus of elasticity similar to human vertebral bone. The Porous Layers on the endplate contact surfaces and along the vertical walls of the graft window utilize Pore Matrix Technology, a geometry that unlike smooth PEEK, is designed to mimic anatomical cancellous bone, with interconnected pores. The surface porosity is designed to promote cell signaling, on-growth, in-growth, and fusion. The HAcancellous PEEK-C Implant may provide an increased opportunity for bone ingrowth and for achieving early integration3. In vitro performance or animal studies may not be representative of clinical performance.
Innovasis will be showing HAcancellous™ PEEK-C at the upcoming NASS meeting (October 12-15) in Chicago at booth #3924.
“We are very excited for this next evolution in interbody fusion devices. To be able to construct an implant similar to cancellous bone is a major achievement. We are seeing amazing results from our current Bioactive implants with fusion rates and patient satisfaction. Being able to add the next evolution of Bioactive implants to our BioBase® registry is very exciting!”- Russ Whetton, Director of Marketing and Education at Innovasis.
The BioBase Data Registry is a multicenter, observational, quality-assessment repository. It is sponsored by Innovasis as part of our commitment to quality and patient outcomes. This program allows surgeons to collect patient outcomes and fusion data in a secure, HIPAA-compliant database that is accessed on a real-time basis.
During this year’s annual ISASS meeting, Georgetown University (MedStar) referenced results from the BioBase Data Registry. In the cervical arm of the registry across 50 sites, 33.1% of patients had 1 level fused; 36.1%- 2 levels, 22.1%- 3 levels, and 8.4%- 4 levels. Fusion was confirmed in 96% of all levels at 12 months using a rotational ROM method. VAS neck (62.5 to 22.4) and arm (39.7 to 15.7) both decreased at 12 months (p < 0.01). At 12 months 97.3% of patients were satisfied or somewhat satisfied.
About Innovasis:
Innovasis, Inc. is a rapidly growing company engaged in the research, development, manufacturing, and marketing of spinal implant devices and related products. Innovasis offers a spinal product line with implants and instruments that address the major pathologies and focus areas of traditional spinal surgery. Innovasis is fully committed to providing surgeons and distributors with training, support, and excellent customer service, thus ensuring the establishment of a strong and long-term strategic partnership.
For information regarding Innovasis please email marketing@innovasis.com