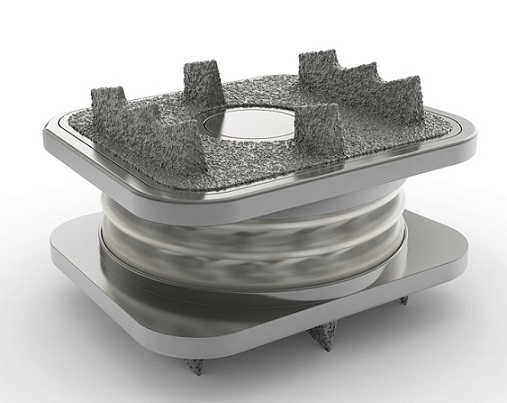
CARLSBAD, Calif., Oct. 06, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for the patented minimally invasive SiLO TFX MIS Sacroiliac Joint Fixation System. The Aurora Spine SiLO TFX MIS Sacroiliac Joint Fixation System, is intended for sacroiliac joint fusion for conditions including sacroiliac joint disruptions and degenerative sacroiliitis. The SiLO TFX MIS Sacroiliac Joint Fixation System includes a Transfixing-Cone, an ilium screw, a sacrum screw and associated instrumentation. The SiLO TFX implants are designed to transfix the sacrum and ilium, providing stability for bony fusion.
“We are pleased with our continued commercial momentum as we expand our footprint for providing differentiated surgical devices in the sacropelvic/sacroiliac space,” said Trent Northcutt, President, and Chief Executive Officer. “We will make meaningful investments in our sales force and physician training, which we believe will enable us to further capitalize on the growth in outpatient surgery centers across the United States.”
Mr. Laszlo Garamszegi, Chief Technology Officer of Aurora Spine, added, “We are excited about this patented game-changing technology. Increased awareness of SI joint disruption as a significant contributor to back pain led us to develop a minimally invasive solution to treat the condition. It is a breakthrough for Aurora to offer a titanium version of our SiLO family of products, which offers additional fixation options to transfix the joint.”
“This is a significant milestone in the evolution of the treatment of SI joint dysfunction and clearly sets SiLO TFX apart from any other surgical options. This patented system transfixes the sacrum and ilium to provide superior clinical outcomes,” said Dr. Michael Stoffman, Neurosurgeon at the University at Buffalo Neurosurgery. “The SiLO TFX clearance is an essential milestone for Aurora Spine as they are committed to helping patients experiencing chronic SI Joint pain.”
About Aurora Spine
Aurora Spine is focused on bringing new solutions to the spinal implant market through a series of innovative, minimally invasive, regenerative spinal implant technologies. Additional information can be accessed at www.aurora-spine.com or www.aurorapaincare.com.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Statements
This news release contains forward-looking information that involves substantial known and unknown risks and uncertainties, most of which are beyond the control of Aurora Spine, including, without limitation, those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Information” in Aurora Spine’s final prospectus (collectively, “forward-looking information”). Forward-looking information in this news release includes information concerning the proposed use and success of the company’s products in surgical procedures. Aurora Spine cautions investors of Aurora Spine’s securities about important factors that could cause Aurora Spine’s actual results to differ materially from those projected in any forward-looking statements included in this news release. Any statements that express, or involve discussions as to, expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and may be forward-looking and may involve estimates, assumptions and uncertainties which could cause actual results or outcomes to differ unilaterally from those expressed in such forward-looking statements. No assurance can be given that the expectations set out herein will prove to be correct and, accordingly, prospective investors should not place undue reliance on these forward-looking statements. These statements speak only as of the date of this press release and Aurora Spine does not assume any obligation to update or revise them to reflect new events or circumstances.
Contact:
Aurora Spine Corporation
Trent Northcutt
President and Chief Executive Officer
(760) 424-2004
Chad Clouse
Chief Financial Officer
(760) 424-2004
Adam Lowensteiner
LYTHAM PARTNERS, LLC
Phoenix | New York
Telephone: 646-829-9700
asapf@lythampartners.com