Dr. J. Alex Sielatycki, SOSI Spine Surgeon; Jessica Nyquist, SOSI Physician Assistant; and Dr. Scott Hodges, 3Spine Medical Director performed the first Balanced Back Total Joint Replacement clinical trial case in Colorado on October 11, 2022. (PRNewsfoto/Steamboat Orthopaedic & Spine Institute)
Steamboat Springs is the first clinical site in the western US for the revolutionary alternative to spine fusion
STEAMBOAT SPRINGS, Colo., Oct. 19, 2022 /PRNewswire/ — Steamboat Orthopaedic & Spine Institute has completed its first series of cases in the BalancedBack Total Joint Replacement pivotal clinical trial using the breakthrough 3Spine MOTUS lumbar device, which is an alternative to spine fusion surgery. J. Alex Sielatycki, MD of Steamboat Orthopedic and Spine Institute and UC Health Yampa Valley Medical Center completed the surgeries at the Steamboat Surgery Center. The Steamboat Surgery Center is jointly run by UC Health and Steamboat Orthopaedic & Spine Institute and is the first BalancedBack clinical site in the western US.
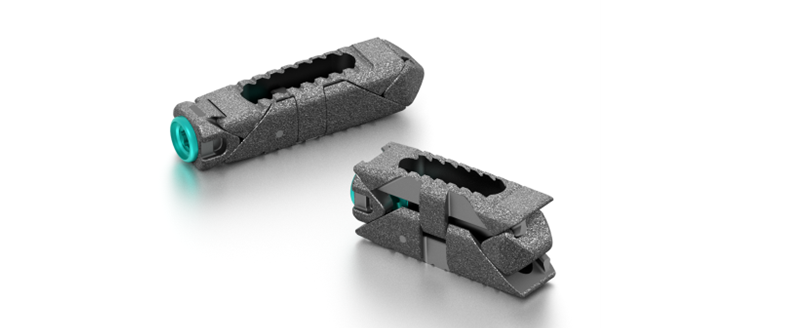
3Spine’s MOTUS device, the implant used in the BalancedBack Total Joint Replacement procedure, is a ‘first of its kind’ technology replacing the function of the disc and facet joints through a posterior approach. The procedure is intended to broadly address leg pain, back pain, and spinal instability, while correcting posture and restoring freedom of movement through reconstruction of the functional spinal unit.
“This motion preserving lumbar joint replacement is truly the first of its kind and has the potential to dramatically change the way spine care is provided not only in this country but throughout the world,” said Dr. Sielatycki. “With the MOTUS total joint replacement technology, I believe we have an answer to the decades-long problem of lumbar fusion complications which have plagued so many patients.”
Curtis Mayse, CEO of the Steamboat Orthopaedic & Spine institute said, “Low back issues are incredibly common. More than 80% of us will experience leg and/or back pain in their lifetime, and we all know someone who has had a poor outcome with spinal fusion. All of us, particularly our active patient population in the Steamboat area, are looking for a better solution, and we are thrilled to participate in this breakthrough trial. I congratulation Dr. Sielatycki and the surgical team on their achievement.”
3Spine, Inc., the study sponsor, is seeking single-level indications from L1-S1 in patients suffering from lumbar degeneration with or without foraminal or recess spinal stenosis with no more than a grade 1 spondylolisthesis. Additional details are available at ClinicalTrials.gov. Patients interested in participating in the clinical trial in Steamboat Springs, Colorado can email jforcum@steamboatortho.com for more information.
Media Contact:
Paige Boucher
paige@insideout-pr.com
SOURCE Steamboat Orthopaedic & Spine Institute