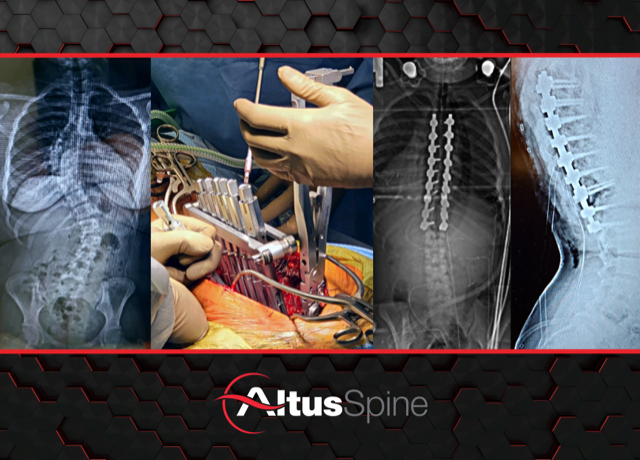
West Chester, PA – February 7, 2023 /OrthoSpineNews/ – Altus Spine, a leader in the development and innovation of medical devices used in spinal correction surgery, announces an important evolutionary milestone for its popular Monaco Pedicle Screw System. With the release of advanced reduction and correction instrumentation, and specialized implant technologies, the Monaco Pedicle System was recently used to perform its first complex deformity procedure at NYU Medical Center.
Dr. Anthony Petrizzo, Co-Chief, Division of Spine Surgery, NYU Langone Long Island School of Medicine, performed the first surgery along with Dr. Themistocles Protopsaltis, Chief, Division of Spine Surgery, and Department of Orthopedic Surgery at NYU Langone. Dr. Petrizzo comments, “The Monaco rod reducers and correction instruments worked flawlessly. The cobalt chrome screw head allows for very secure connections with attachments providing for powerful correction and reduction of complex deformities. I’m very pleased with the case results and I’m looking forward to the next procedures.” Dr. Protopsaltis adds, “The new Monaco instruments helped immensely during the reduction and de-rotation phase of surgery. With the Monaco system’s 6.0mm cobalt chrome rodding, we feel comfortable that the correction construct is adequately rigid and stable.”

“This important milestone for the Monaco System resulted from the culmination of tireless collaboration between experienced clinical minds and Altus engineering innovation,” stated Michael Fitzgerald, President of Altus Spine. “We are so very happy to have contributed in some small way to improving another patient’s quality of life. And, we are driving to give Monaco best-in-class capabilities to allow it to successfully perform the most difficult long segment complex procedures.”
The Monaco Pedicle Screw System provides the market with an ultra-low profile screw head, manufactured from Cobalt Chrome that accommodates Ø5.5 & Ø6.0mm Cobalt Chrome and Titanium alloy rods. The bone screw thread design combines a single to dual lead thread design with a dual core diameter optimizing thread purchase. A growing menu of specialized implants provides the clinician with abundant options in treatment. The implants are paired with a streamlined set of instruments designed to provide intra-operative flexibility.
Altus Spine
Based in West Chester, PA, Altus Spine is dedicated to creating the next generation of medical devices. Altus strives to improve patient care by designing and manufacturing products to meet the highest standards in an ever changing and evolving field. Implemented and used by over 100 hospitals across the United States, Altus is among the fastest growing spinal implant companies in the world. For additional information, or to inquire about distribution opportunities, please visit www.altus-spine.com.
Forward Looking Statements
All statements made in the above press release, with the exception of historical fact, may be forward[1]looking statements that include risk, uncertainty, and assumptions. These factors could cause Altus Spine’s results to differ from those predicted if they do not occur as expected. These uncertain factors include, but are not limited to: acceptance and clearance of the company’s surgical products and procedures, development of new products and procedures, innovations and alterations to existing products and procedures, clinical and statistical verification of the success using Altus Spine’s products, company’s ability to maintain and monitor inventory as it releases new products, its ability to hire and retain personnel, and any other risks stated in prior or subsequent news releases. All risks, indications for use, and potential complications can be found in our most recent 510(k) report from the Food and Drug Administration (FDA). Given the constantly changing market, readers are encouraged to not place undue reliance on forward-looking statements. Altus Spine assumes no obligation to update forward-looking statements as these changes occur, or as events and circumstances are altered, after the date they are posted.