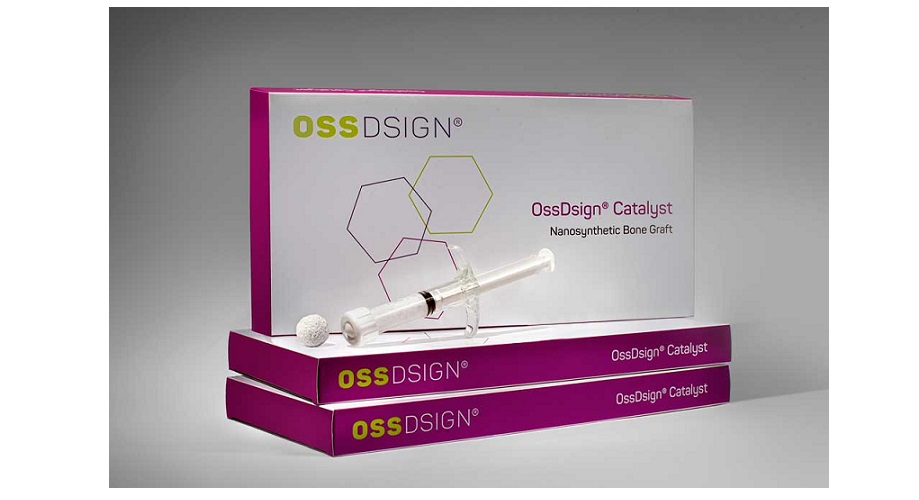
Uppsala, May 9, 2023 – OssDsign AB (publ.) today announces that 1,000 patients have been treated in the U.S. with the innovative nanosynthetic bone graft OssDsign Catalyst. Product awareness is increasing considerably, which is reflected in the rapidly growing number of patients treated.
OssDsign Catalyst has been very well received in the U.S. market since its launch in August 2021. In January OssDsign reported that 500 patients had been treated since launch, and at the beginning of May the number increased to 1,000 patients.
“OssDsign Catalyst is growing exponentially driven by a continuously growing customer base as well as broader usage within the hospitals and, in the last four months alone, we have doubled the number of patients treated. This is an outstanding development that highlights the potential for OssDsign Catalyst to become the preferred product for spinal fusion surgeries,” said Morten Henneveld, CEO of OssDsign.
OssDsign Catalyst is a nanosynthetic bone graft that stimulates the formation of healthy bone tissue in spinal fusion surgeries. The graft is composed of a proprietary nanocrystalline structure which is resorbed and replaced by new and healthy bone tissue. The product was launched in the U.S. in August 2021. The market clearance in the U.S. is based on preclinical results that surpass what is typically seen with other synthetic bone grafts in the most demanding preclinical model for spinal fusion – the Boden model. OssDsign continues to accelerate a robust program of gathering clinical evidence anchored by PROPEL, a U.S.-based multi-center prospective spinal fusion registry, and the clinical study TOP FUSION, in which patient enrolment was completed in April 2022.
For further information, please contact:
Morten Henneveld, CEO, OssDsign AB
Tel: +46 73 382 43 90, email: morten.henneveld@ossdsign.com
Certified Adviser:
Erik Penser Bank AB is the company’s Certified Adviser. Contact information: Erik Penser Bank AB, Box 7405, 103 91 Stockholm, Sweden, phone: +46 (0)8-463 80 00, email: certifiedadviser@penser.se
About OssDsign
OssDsign is a developer and global provider of next generation bone replacement products. Based on cutting edge material science, the company develops and markets products that support the body’s own healing capabilities and thereby improve the clinical outcome in a wide range of orthopedic areas with high medical needs. With a product portfolio consisting of patient-specific implants for cranial surgeries and an off-the-shelf synthetic bone graft for spine surgeries, OssDsign give patients back the life they deserve. The company has a strong commercial presence in the U.S., Europe and selected Asian countries. OssDsign’s share is traded on Nasdaq First North Growth Market in Stockholm, Sweden.