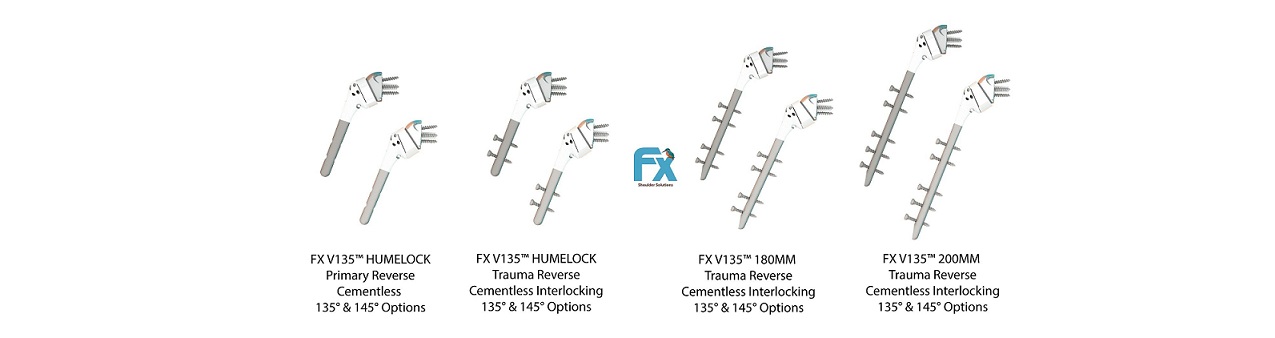
The First-and-Only Interlocking Shoulder System with Intraoperative Options Between 135° and 145° for Reverse Total Shoulder Arthroplasty

DALLAS, June 8, 2023 /PRNewswire/ — FX received FDA clearance to launch the next revolution of their distal interlocking stems. FX adds the lengths 120mm, 180mm and 200mm cementless interlocking stems to further complement the previously cleared 70mm (not interlocking) FX V135™ mini. FX now offers a complete range of sizes (120, 180 and 200mm) for their interlocking humeral stems with intraoperative options for the surgeon to decide between 135° and 145° neck shaft angle in the reverse construct. Surgeons have this choice, even at the last minute before reducing the joint, to be able to change the neck shaft angle, due to the FX unique-to-market net shape molded humeral cups. FX has established itself with its unique-to-market portfolio in the US featuring their previously cleared Humelock Reversed® and Humelock II® shoulder systems with published data to demonstrate the efficacy and advantage that these interlocking cementless stems provide. To see these publications and learn more, please visit our website www.fxshouldersolutions.com.
“We anticipate these additions to our portfolio will generate a lot of excitement as we bring additional interlocking cementless humeral stems to a complex and challenging corner of the shoulder market”, said Baptiste Martin (CEO). He continued to say, “when you add to that the intraoperative option to decide between 135° and 145° by simply changing the one-piece FX humeral component, it is truly a shoulder system like no other on the market. We continue to stay true to our identity as shoulder specialists focused exclusively and solely on shoulder arthroplasty. This additional 510k clearance is really a game changer for the shoulder market and for the surgeon to be able to treat their patients differently.”
The 120mm FX V135™ Humelock allows surgeons to use 135° or 145° neck shaft angle on their primary reverse procedures as well as their 3-4 parts fractures with the same stem. This interlocking stem is completely unique in the shoulder arthroplasty market as no other company can provide this option.
The 180 and 200mm FX V135™ interlocking humeral long stems are the first-to-market and expand the FX portfolio to provide cementless stems for complex fractures. Likewise, these stems may be used in a revision setting providing a novel shoulder solution to surgeons.
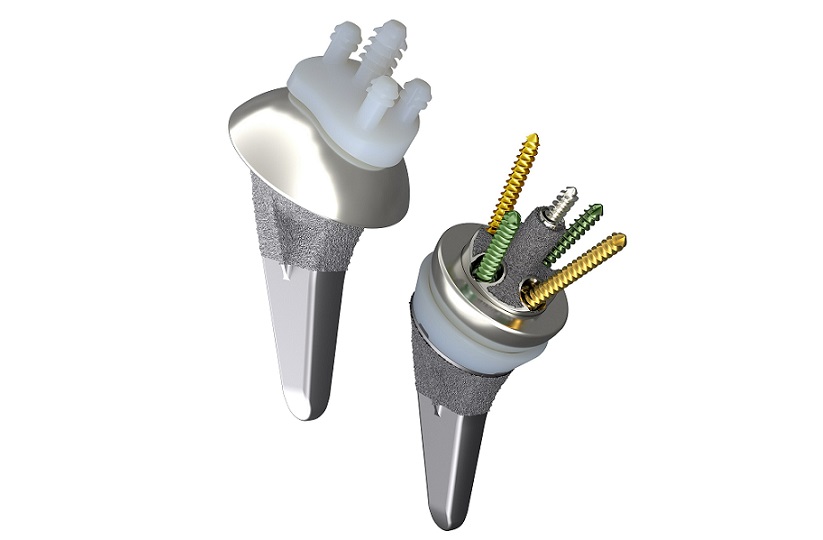
With the FX V135™ shoulder system, the surgeon may trial on the broach as well as the definitive stem at both 135° and 145° neck shaft angles.
The additional FX V135™ stems feature polished suture holes proximally for soft tissue repair.
The FX glenoid reverse system remains the same along the portfolio with a total of 12 baseplate options and 6 different glenosphere options (sizes 32, 36 and 40mm in centered or eccentric styles).
The FX V135™ shoulder system offers to the surgeon wide ranging options to create a unique solution that adapts the system to the patient’s anatomy rather than making the patient conform to the components.
FX has a US headquarters in Dallas, Texas and an OUS headquarters in Viriat, France.
For further information, please contact info@fxshouldersolutions.com or info@fxshouldersolutions.fr.
FX-USA. Phone 1-800-280-0775, Fax 1-800-429-8965.
Photo – https://mma.prnewswire.com/media/2096240/FX_V135.jpg
Logo – https://mma.prnewswire.com/media/2096239/FX_ShoulderSolutions_Logo.jpg
SOURCE FX Solutions