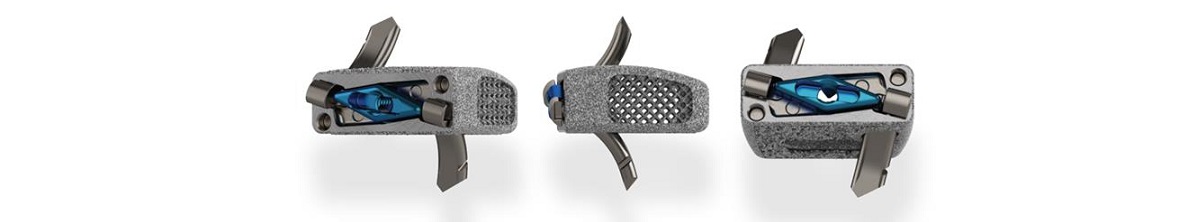
STOCKHOLM, SWEDEN, September 19, 2023 – Karolinska Development AB (Nasdaq Stockholm: KDEV) announces that its portfolio company OssDsign has received clearance from FDA for the use of OssDsign Catalyst in interbody cages in spinal surgery. The FDA clearance creates possibilities in a new indication with major market potential for the company’s innovative nanosynthetic bone graft.
The clearance from the U.S. Food and Drug Administration (FDA) allows surgeons to use the OssDsign Catalyst on-label in any interbody cage cleared for use with synthetic bone grafts. OssDsign Catalyst is the first synthetic bone graft to be cleared to market for interbody use based on bone graft data alone.
The decision by the FDA is based on OssDsign’s outstanding bone regeneration results which surpass other synthetic bone grafts in challenging evaluation models.
“There is a huge demand for the use of synthetic bone graft in interbody cages, assumed to represent as much as 50 percent of all use of bone graft in spinal surgeries. The FDA clearance marks a great opportunity for our portfolio company to expand its business and accelerate its commercialization in the U.S further,” says Viktor Drvota, CEO, Karolinska Development.
Karolinska Development’s shareholding in OssDsign, including indirect ownership via KCIF Co-Investment Fund, amounts to 10.4 percent.
OssDsign is a developer and global provider of next-generation bone replacement products. Based on cutting-edge material science, the company develops and markets products that support the body’s own healing capabilities and thereby improve the clinical outcome in a wide range of orthopedic areas with high medical needs. With a product portfolio consisting of patient-specific implants for cranial surgeries and an off-the-shelf synthetic bone graft for spine surgeries, OssDsign gives back patients the life they deserve. The company has a strong commercial presence in the US, Europe and selected Asian countries. OssDsign’s share is traded on Nasdaq First North Growth Market in Stockholm, Sweden.
For further information, please contact:
Viktor Drvota, CEO, Karolinska Development AB
Phone: +46 73 982 52 02, e-mail: viktor.drvota@karolinskadevelopment.com
Johan Dighed, General Counsel and Deputy CEO, Karolinska Development AB
Phone: +46 70 207 48 26, e-mail: johan.dighed@karolinskadevelopment.com
TO THE EDITORS
About Karolinska Development AB
Karolinska Development AB (Nasdaq Stockholm: KDEV) is a Nordic life sciences investment company. The company focuses on identifying breakthrough medical innovations in the Nordic region that are developed by entrepreneurs and leadership teams. The Company invests in the creation and growth of companies that advance these assets into commercial products that are designed to make a difference to patients’ lives while providing an attractive return on investment to shareholders.
Karolinska Development has access to world-class medical innovations at the Karolinska Institutet and other leading universities and research institutes in the Nordic region. The Company aims to build companies around scientists who are leaders in their fields, supported by experienced management teams and advisers, and co-funded by specialist international investors, to provide the greatest chance of success.
Karolinska Development has a portfolio of eleven companies targeting opportunities in innovative treatment for life-threatening or serious debilitating diseases.
The Company is led by an entrepreneurial team of investment professionals with a proven track record as company builders and with access to a strong global network.
For more information, please visit www.karolinskadevelopment.com.