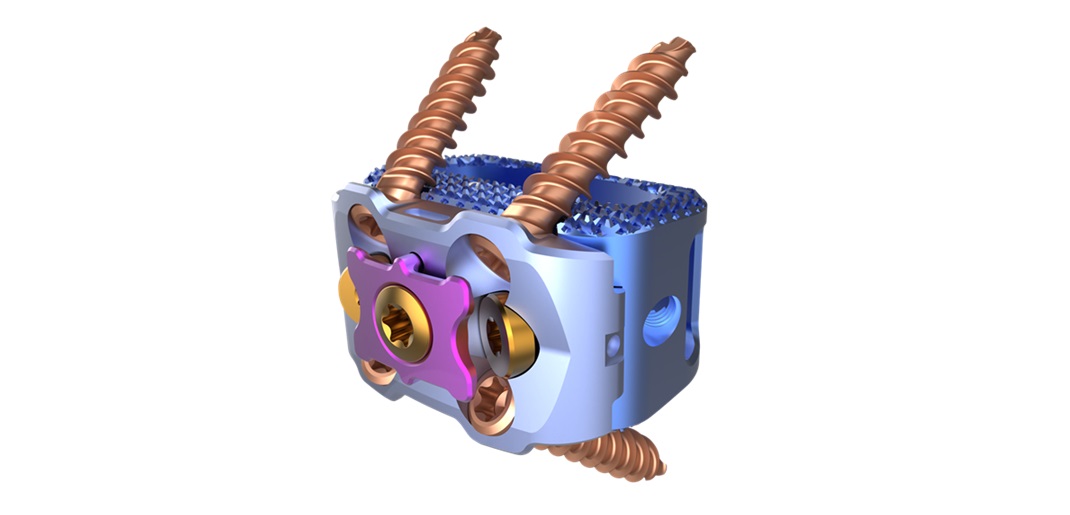
KeyLift is the fifth FDA-approved product developed by FloSpine, all invented at the Research Park at Florida Atlantic University®
BOCA RATON, FLORIDA, USA, November 1, 2023 / EINPresswire.com / — Together with FloSpine, the Research Park at Florida Atlantic University® is pleased to announce that FloSpine has achieved 510(k) clearance for its KeyLift™ Expandable Interlaminar Stabilization System from the FDA, and has expanded out of the Global Ventures entrepreneur support center into a new, larger office to support its growth.
The newly approved device addresses spinal stenosis, a common and debilitating condition that affects more than 100 million people worldwide, resulting in approximately 600,000 surgeries annually in the United States alone. It occurs when the spinal canal narrows, causing pressure on the nerves and leading to severe back and leg pain, weakness, and numbness. Traditional treatment options for spinal stenosis have often involved invasive surgical procedures with lengthy recovery times. The KeyLift system introduces a less invasive alternative, enabling spine and interventional pain physicians to administer this revolutionary treatment in an outpatient surgery center.
The KeyLift Expandable Interlaminar Stabilization System features an innovative, expandable design that allows for precise, patient-specific surgical intervention. Unlocking the biomechanics of the spine, KeyLift offers three key advantages:
1. Support – KeyLift provides support at the strongest part of the spinal column, the lamina. This distinguishes it from existing devices that only support the spinous process, which is troublesome in patients with osteoporosis.
2. Lift – With the capacity to expand up to 4mm in height, KeyLift effectively distracts and relieves nerves, alleviating pain caused by spinal stenosis.
3. Stabilize – Positioned closer to the spine’s instantaneous axis of rotation, KeyLift promotes disc health by balancing the intervertebral discs.
This groundbreaking technology not only reduces patient discomfort and pain but also significantly shortens recovery times by being performed in an outpatient surgery center, allowing patients to return to their daily lives more quickly.
The KeyLift system’s expandable design is the result of years of research, development, and rigorous testing by the FloSpine team, anchored by Luis Escobar, lead design engineer.
“KeyLift provides a more mechanically stable design in a minimally invasive package,” says Dr. Cheng-Lun Soo, MD, an orthopedic spine surgeon and co-inventor of the KeyLift. “As a spine surgeon of over 25 years, I believe this technology will reach more patients by enabling more interventional physicians to treat mild to moderate spinal stenosis without the fear of a large surgery.”
Like the four other products invented by FloSpine and approved by the FDA, KeyLift has a Florida inspired name. FloSpine is committed to advancing the field of spinal healthcare, and to support its continued growth, it recently expanded its headquarters to a 5,000 square foot facility in the Research Park at Florida Atlantic University, having completed its scale-up at Global Ventures. A majority of FloSpine’s hiring has been from Florida Atlantic University’s College of Engineering, and over the next two years, it plans to increase from 8 employees to 18.
“This is the culmination of years of research and development to deliver a medical device that helps improve the lives of patients suffering from spinal stenosis. Our recent move to larger premises in the Research Park at FAU will allow us to train our surgical and distribution partners to become increasingly competitive in the spinal implant device market,” said Peter Harris, founder and CEO of FloSpine. “This marks two significant achievements for our company as we grow to support our spine devices for both interventional pain physicians and spine surgeons. We are confident that KeyLift will become the standard in treating this condition, offering an overall improved quality of life for patients.”
Andrew Duffell, president of the Research Park at FAU added, “FloSpine’s growth and expansion from Global Ventures into the Research Park is an inspiration to healthtech entrepreneurs and Florida Atlantic students who see their classmates excel in an exciting, growing industry. We very much look forward to supporting the team as it continues to innovate and contribute to the region’s economy.”
In loving memory of Luis Escobar, Senior Design Engineer, and friend.
Andrew Duffell
Research Park at FAU
+1 561-416-6092
email us here
Visit us on social media:
Facebook
LinkedIn
Instagram
YouTube
NOTE: This content is not written by or endorsed by “KLST/KSAN”, its advertisers, or Nexstar Media Inc.
For inquiries or corrections to Press Releases, please reach out to EIN Presswire.