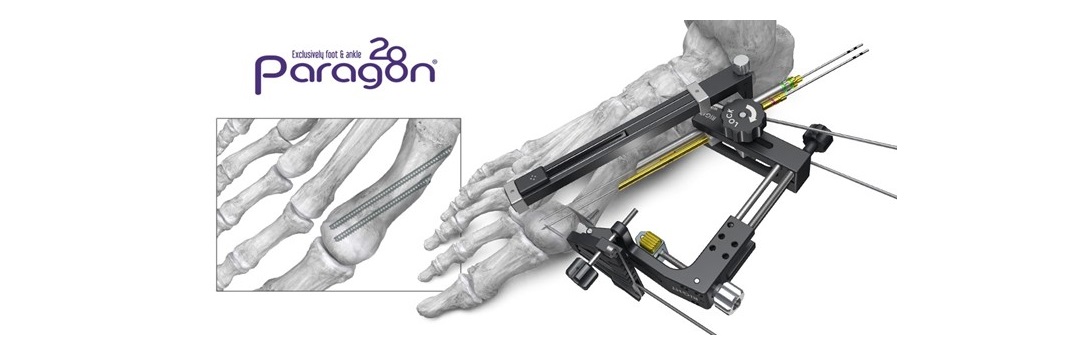
ENGLEWOOD, Colo., January 24, 2024 –(BUSINESS WIRE)– Paragon 28, Inc. (NYSE: FNA) is proud to announce a novel solution in modernizing foot and ankle surgery with the launch of its FJ2000™ Power Console and Burr System designed for a wide range of minimally invasive and open procedures in the foot and ankle.
The FJ2000™ Power Console and Burr System is highly versatile and designed for quick and efficient setup at the outset of each procedure. The System has three pre-set options, including a low speed, high torque setting designed to help reduce thermal necrosis during bone and joint preparation and preserve healthy bone healing.
The FJ2000™ offers single-use hand pieces and a substantial selection of burrs and instruments in a sterile packed kit prepared for immediate use. As a single-use option, the FJ2000™ brings greater flexibility and speed to the surgeon while preventing costly delays due to wear and tear and sterilization associated with traditional power systems.
Prof. Dr. med. Hazibullah Waizy, commented, “This system is very efficient from a workflow perspective. The combination of adjustable speed and power settings and drilling function allows surgeons to work precisely in a way most suitable for each unique procedure. The sterile packed, single-use hand pieces and burrs are dependable and allow me to turn cases quickly and effectively without waiting for other instruments to become available.”
Paragon 28’s CEO, Albert DaCosta, commented, “The FJ2000™ brings several benefits to the foot and ankle community, ranging from health system cost, operating room efficiency, surgeon optionality and ultimately improved patient outcomes. We are incredibly proud to bring a modernized approach to the market that is truly differentiated in so many ways.”
The FJ2000™ Power Console and Burr System bolsters Paragon 28’s bunion and forefoot solutions offering, which includes the Gorilla® Precision® Lapidus Plate, Phantom® Small Bone Intramedullary Nail, Phantom® MIS System, Gorilla® Precision® MTP Plate, Phantom® Metatarsal Shortening System, TenoTac®, Paratrooper™, and HammerTube™. With this comprehensive portfolio, Paragon 28® provides its customers with a single source to address their bunion and forefoot needs.
About Paragon 28, Inc.
Based in Englewood, CO., Paragon 28, is a leading medical device company exclusively focused on the foot and ankle orthopedic market and is dedicated to improving patient lives. From the onset, Paragon 28® has provided innovative orthopedic solutions, procedural approaches and instrumentation that cover a wide range of foot and ankle ailments including fracture fixation, forefoot, ankle, progressive collapsing foot deformity (PCFD) or flatfoot, Charcot foot and orthobiologics. The company designs products with both the patient and surgeon in mind, with the goal of improving outcomes, reducing ailment recurrence and complication rates, and making the procedures simpler, consistent, and reproducible.
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward-looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward-looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the fiscal year ended December 31, 2022 and its Quarterly Reports on Form 10-Q, as updated periodically with its other filings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Disclaimer
Prof. Dr. med. Waizy may report consulting and royalty fees from Paragon 28 in connection with the provision of product development services to Paragon 28.
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.
Contacts
Investor Contact
Matthew Brinckman
Senior Vice President, Strategy and Investor Relations
Phone: (720) 912-1332
mbrinckman@paragon28.com