CARLSBAD, Calif., August 5, 2024–(BUSINESS WIRE)–Carlsmed, the leader in personalized spine surgery, announced today that in the Hospital Inpatient Prospective Payment System Final Rule for fiscal year 2025, the Centers for Medicare & Medicaid Services (CMS) assigned spinal fusion cases with aprevo® custom-made anatomically designed interbody fusion devices to the highest level of the new spinal fusion Medicare Severity-Diagnosis Related Groups (MS-DRGs) it created. The new assignments and payment rates are favorable and will go into effect on October 1, 2024.
“Carlsmed has revolutionized the standard of care for spine surgery with our unique patient-centric approach to implant design,” states Mike Cordonnier, CEO of Carlsmed. “We appreciate CMS’s thoughtful reimbursement decision to ensure Medicare beneficiary access to this important technology after the New Technology Add-on Payment (NTAP) status concludes.”
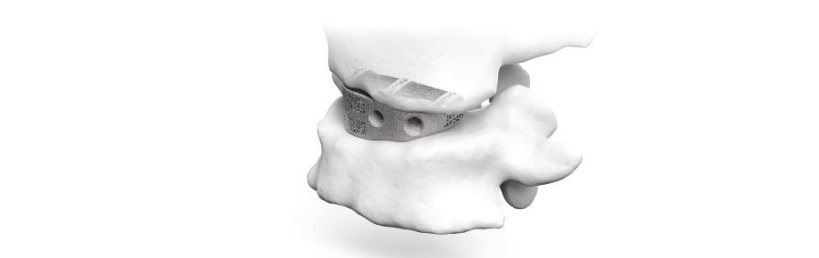
Leveraging its AI-enabled proprietary software, Carlsmed designs each aprevo® implant to match the patient’s unique anatomy. The aprevo® implants are built on demand and delivered sterile packed for surgery.
Earlier this year, Carlsmed released clinical data on its aprevo® custom-made anatomically designed devices. The data, which was published in the Global Spine Journal, concluded that the use of aprevo® devices in spine surgery enabled an improvement in achieving post-operative alignment. Prior studies have shown that achieving optimal alignment during spine fusion surgery is highly correlated to reduced complications.
Carlsmed’s full suite of custom interbody devices for lumbar surgery are available in the U.S. The Company has received Breakthrough Device designation for cervical use and anticipates the launch of cervical custom-made interbody devices by the end of 2025.
About Carlsmed
The Carlsmed aprevo® personalized surgery platform is designed to improve the standard of care for spine surgery one patient at a time. Carlsmed’s implantable devices and software platforms are FDA cleared for lumbar spine fusion.
Contacts
For media and investor inquiries, please contact Jodi Allen at jodi@carlsmed.com