Salt Lake City, Utah, August 20, 2024 /OrthoSpineNews/ – Innovasis Inc., a leader in spinal innovation and research, is pleased to announce the latest findings from The BioBase® Data Registry, which now has data from over 3,800 procedures. Research conducted by Kazarian, MD et al. at the Hospital for Special Surgery, NY, and recently published in the Global Spine Journal, provides significant insights into the effectiveness of various lumbar interbody fusion (LIF) procedures using HA-Infused PEEK and HA-Treated Titanium Alloy interbody cages.
The retrospective observational study involved 54 patients who underwent single-level anterior (ALIF), thoraco-LIF (TLIF), posterior LIF (PLIF), and lateral LIF (LLIF) procedures. The results demonstrate notable improvements in patient outcomes and range of motion (RoM) metrics, highlighting the success and efficacy of Innovasis’ advanced spinal fusion technologies.
Key Findings:
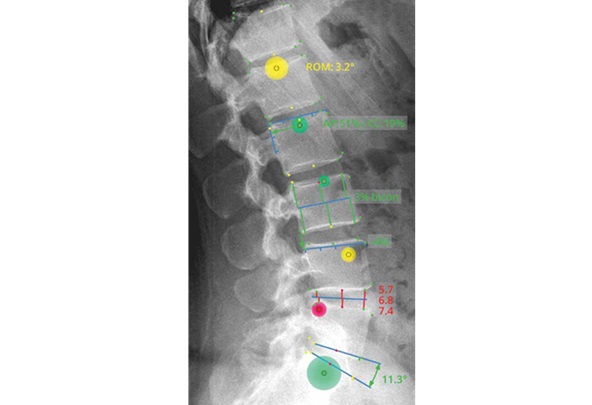
– Overall Lumbar RoM: The study reports a 14% decrease in overall range of motion from L1-S1, equating to a minimal loss of only 3.3 degrees post-surgery.
– Index Level Segmental RoM: A significant reduction of 77% in segmental RoM at the index level was observed, indicating effective stabilization of the targeted spinal segment.
– Adjacent Segment RoM: The cranial adjacent level segments showed a 34% increase in RoM, while caudal level changes varied, underscoring the dynamic biomechanical adjustments following surgery.
– Cluster Analysis: Detailed cluster analysis by fusion level (L3-4, L4-5, L5-S1) revealed that cranial adjacent segments typically increased in RoM, caudal segments exhibited variable changes, and index levels consistently decreased significantly.
– Successful Fusion Rates: Successful fusion was verified in 96% of all instrumented segments, reflecting the high efficacy of the HA PEEK and HA-Treated Titanium Alloy cages used in these procedures.
Overall Conclusions:
This comprehensive study helps to verify the efficacy of Innovasis’ unique HA PEEK and HA-Treated Titanium Alloy interbody cages. It demonstrates significant positive changes in both range of motion (RoM) and patient-reported outcome measures (PROMs).
About Innovasis Inc.:
Innovasis Inc. is a pioneering medical device company dedicated to the research, development, and commercialization of products for the treatment of spinal disorders. With a commitment to innovation and excellence, Innovasis aims to improve patient care and outcomes through advanced technology and rigorous clinical research.
Contact Information
For more information about The BioBase® Registry or to participate in the program, please contact info@innovasis.com