Approval underscores Cerapedics’ commitment to investing in evidence that brings scientific rigor to bone grafting for spinal fusion.
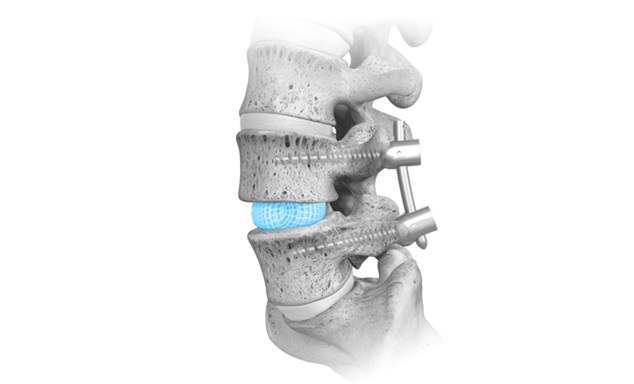
WESTMINSTER, Colo., Sept. 24, 2024 /PRNewswire/ — Cerapedics Inc., a global, commercial-stage orthopedics company dedicated to redefining the standard of care for bone repair, today announced the U.S. Food and Drug Administration (FDA) approval for an expansion to the indications for use and labeling for i-FACTOR P-15 Peptide Enhanced Bone Graft. With this label expansion, i-FACTOR is now approved for single-level anterior cervical discectomy fusion (ACDF) with an allograft bone ring, or in conjunction with a PEEK, titanium alloy or PEEK/titanium fusion interbody device cleared by the FDA for use in the cervical spine, and with supplemental anterior plate fixation.
The premarket approval (PMA) investigational device exemption (IDE) study for i-FACTOR was designed in 2005, using the gold standard for ACDF procedures at that time – an allograft ring. The practice of medicine has evolved in the subsequent years, and this indication expansion underscores Cerapedics’ commitment to invest in evidence that supports clinical practice and meets the needs of our physician customers. i-FACTOR can now be used with most interbody cages on the market providing proven safety and fusion efficacy through human clinical data.
“This label expansion approval is part of our deep commitment to bring scientific rigor to bone grafting so surgeons and the patients they treat have access to products with proven safety, efficacy and evidence,” said Valeska Schroeder, Chief Executive Officer of Cerapedics. “i-FACTOR has been a trusted choice in bone graft selection in the United States since 2015 as the only bone graft replacement FDA-approved for single-level ACDF procedures with a premarket approval based on a Level 1 human clinical trial. We are proud to have FDA approval for the safe and effective use of i-FACTOR in PEEK and titanium cages.”
ACDF is a surgical procedure to reduce or eliminate pain because of issues in the neck like degenerative disc disease by removing a damaged disc and then uniting (fusing) the spinal vertebral bones above and below the damaged disc to create spinal stability along with decompression of the spine. The fusion is achieved using a bone graft, which over time, grows together with the vertebrae to form one bone. In 2023, there were an estimated 465,000 spinal fusion cases that used a bone graft replacement in the U.S.1
About i-FACTOR P-15 Peptide Enhanced Bone Graft
i-FACTOR is a Class III FDA approved bone graft backed by rigorous Level 1 human clinical data from an IDE study published in Spine and Neurosurgery. It is the only spinal bone graft powered by P-15 Osteogenic Cell Binding Peptide, with a precise bone-building mechanism.* i-FACTOR has a demonstrated safety profile and is as safe as local autograft in single-level ACDF with proven statistical superiority in overall success at one and two years.**
i-FACTOR Peptide Enhanced Bone Graft is indicated for use in skeletally mature patients for reconstruction of a degenerated cervical disc at one level from C3-C4 to C6-C7. i-FACTOR Peptide Enhanced Bone Graft must be used inside an allograft bone ring, or in conjunction with a PEEK, titanium alloy or PEEK/titanium fusion interbody device cleared by the FDA for use in the cervical spine, and with supplemental anterior plate fixation.
For more information, full description of the indication for use, contraindications, safety warnings, etc. about i-FACTOR, please visit our website.
* Surface-bound mechanism of action
** Overall success is defined as all four endpoints being met including: Fusion, Function (NDI), Neurological, Safety as demonstrated in the single-level ACDF PMA
About Cerapedics
Cerapedics is a global, commercial-stage orthopedics company that aspires to redefine the standard of care for bone repair, healing bones faster and at higher rates, without compromising safety, so that patients can live their healthiest lives. Bone grafts, including Cerapedics’ products, are used in over four million annual spine, orthopedics, trauma, and interventional procedures worldwide. Cerapedics is headquartered in Westminster, CO.
For more information, visit us at www.cerapedics.com.
Media contact: FleishmanHillard
fh-cerapedics@fleishman.com
1 Clarivate | Decision Resources Group. Bone Graft Substitutes. December 2022.
SOURCE Cerapedics Inc.