Spine tech innovators elliquence and Implanet to exhibit at NASS in Chicago, September 25-28, 2024 (elliquence LLC Booth 2419 Implanet Booth 2412)
September 25, 2024 /OrthoSpineNews/ – elliquence LLC, specializing in advanced minimally invasive surgical technologies, and Implanet (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME), a medical technology company specializing in implants for orthopedic surgery and the distribution of advanced medical equipment, have jointly announced a strategic agreement for distribution of Implanet’s Ultrasonic Bone Scalpel in endoscopic spine surgery. The announcement is being made at the North American Spine Society (NASS) Annual Meeting, where both companies are showcasing their latest technologies.
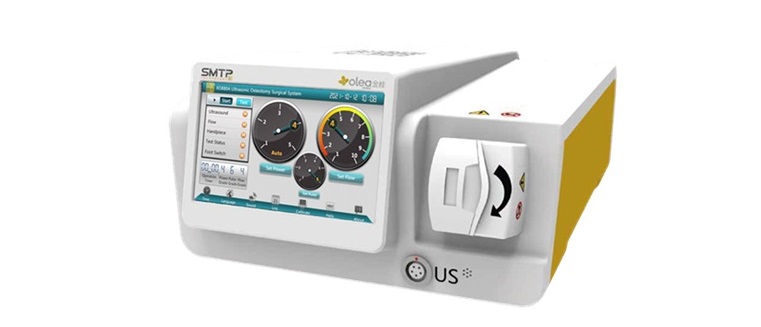
elliquence’s Surgi-Max Ultra platform is widely recognized for delivering effective soft tissue dissection and coagulation in endoscopic procedures, while Implanet’s Olea technology enables precise and efficient bone cutting. By combining the precision of Implanet’s ultrasonic bone scalpel with elliquence’s well-established Surgi-Max Ultra radiofrequency and its bipolar Trigger-Flex technology, surgeons are now equipped with a unique synergy of bone and soft tissue management tools. Integrating the ultrasonic bone scalpel allows for safer, more controlled bone removal, preserving neural structures and minimizing soft tissue damage—further enhancing the precision and outcomes of minimally invasive spine surgery.
“We’re honored to be engaging with our like-minded colleagues to further introduce the surgeon community to first-of-its-kind endoscopic blade technology,” stated Max Painter, Vice President and General Manager of Implanet America. “It’s rewarding to be able to team with other innovation-driven companies to expand the reach of this innovative technology and provide the broader surgeon community with game-changing solutions.”
“This partnership is an endorsement of the clinical value of our technology,” added Ludovic Lastennet, Implanet’s Chief Executive Officer. “This type of partnership fits with the medium-term development plan we announced in the middle of 2023 and the partnership with SanYou Medical. It demonstrates our ability to market new technologies with a newly formed seasoned team in the US. With this team on board, we will be able to launch new mainstream product lines in the short term.”
About elliquence, LLC.
elliquence, LLC is a leading medical device company based in New York, specializing in advanced minimally invasive surgical technologies. With over 50 years of innovation, Elliquence is recognized globally for its Surgi-Max Ultra radiofrequency platform, which provides surgeons with unparalleled precision in soft tissue dissection and coagulation. The company’s mission is to partner with the medical community to develop high-quality, cost-effective solutions that enhance patient outcomes while reducing procedural risks and recovery times. elliquence’s cutting-edge technologies are widely used in endoscopic spine surgery, orthopedics, neurosurgery, and pain management. Known for pushing the boundaries of surgical innovation, Elliquence continues to lead the charge in improving the efficiency and effectiveness of minimally invasive procedures.
For further information, please visit www.elliquence.com
About IMPLANET
Founded in 2007, IMPLANET is a medical technology company that manufactures high-quality implants for orthopedic surgery and distributing medical technology equipment. Its activity revolves around a comprehensive innovative solution for improving the treatment of spinal pathologies (JAZZ®) complemented by the product range offered by Orthopaedic & Spine Development (OSD), acquired in May 2021 (thoraco-lumbar screws, cages and cervical plates). Implanet’s tried-and-tested orthopedic platform is based on the traceability of its products. Protected by four families of international patents, JAZZ® has obtained 510(k) regulatory clearance from the Food and Drug Administration (FDA) in the United States, the CE mark in Europe and ANVISA approval in Brazil. In 2022, IMPLANET entered into a commercial, technological and financial partnership with SANYOU MEDICAL, China’s second largest medical device manufacturer. IMPLANET employs 43 staff and recorded a consolidated revenue of €7.4 million in 2023. Based near Bordeaux in France, IMPLANET opened a US subsidiary in Boston in 2013. IMPLANET is listed on the Euronext Growth market in Paris.
For further information, please visit www.Implanet.com.
USA Media Contact
Paul Williams
310-569-0023
paul@medialinecommunications.com