DUBLIN, October 3, 2024 –( BUSINESS WIRE )– Mainstay Medical announced today that the U.S. Centers for Disease Control and Prevention (CDC) has designated an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnostic code specifically for multifidus dysfunction in the lower back. The new diagnostic code, M62.85: dysfunction of the multifidus muscles, lumbar region, is effective October 1, 2024.
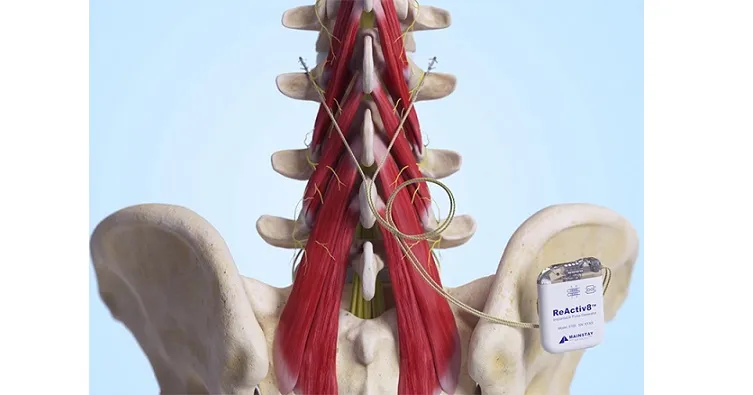
In response to an acute injury to the low back, the brain typically reduces the neural drive that activates the multifidus muscle, resulting in reduced multifidus muscle activity. Since the multifidus muscle is a primary stabilizer of the lumbar spine, this inhibition can lead to joint instability and overload, exacerbating the problem and contributing to chronic low back pain. The ReActiv8® Restorative Neurostimulation™ System is designed to provide electrical stimulation of the nerves activating the multifidus muscle, helping to restore its function and facilitate recovery from chronic low back pain. ReActiv8 is the only FDA-approved treatment for the management of intractable chronic low back pain associated with multifidus muscle dysfunction.
“The CDC has recognized multifidus dysfunction as a significant cause of chronic low back pain and now provides clinicians with a clearer path to specifically diagnose this condition. Multifidus dysfunction is a very specific disease state that does not respond to palliative forms of stimulation, and we are pleased that this new code clearly reflects the underlying disease state,” said Jason Hannon, CEO, Mainstay Medical.
About ReActiv8®
ReActiv8 is an implantable medical device intended for the treatment of adults with chronic intractable low back pain associated with multifidus dysfunction. Multifidus dysfunction can be diagnosed by imaging or physiological testing in adults for whom treatments, including analgesics and physiotherapy, have failed and for whom spinal surgery is not recommended. ReActiv8 has received regulatory approvals in several geographies. The device is available in the European Economic Area, Australia, the United Kingdom and the United States.
About Mainstay Medical
Mainstay Medical is a medical device company focused on commercializing its innovative implantable restorative neurostimulation system, the ReActiv8, for people with chronic, mechanically disabling low back pain. Mainstay Medical is headquartered in Dublin, Ireland, with subsidiaries in Ireland, the United States, Australia, Germany and the Netherlands.
For more details: www.mainstaymedical.com .
Mainstay Forward-Looking Statements
Statements contained in this press release, other than statements of historical fact, are or may be deemed to be forward-looking statements. These forward-looking statements may include, but are not limited to, statements regarding the Company’s current intentions, beliefs or expectations regarding, among other things, the Company’s use of the new ICD-10 code and the Company’s business efforts and performance, results, financial condition, financing strategies, product design and development, intellectual property portfolio and scope, regulatory applications and approvals and reimbursement terms.
Forward-looking statements involve risks and uncertainties and are not guarantees of future performance. Actual results may differ materially from those described or suggested by the forward-looking statements. A number of factors could cause results and developments to differ materially from those expressed or implied by the forward-looking statements in this release, including the risks and uncertainties included in the Company’s Annual Report for the year ended December 31, 2022, which should be read in conjunction with the Company’s public information (available on the Company’s website www.mainstaymedical.com ). Forward-looking statements speak only as of the date of this release.
The text of the press release resulting from a translation should in no way be considered official. The only version of the press release that is authoritative is that of the press release in its original language. The translation must always be compared with the source text, which will constitute precedent.
Contacts
Mainstay, Public Relations and Investor Relations:
LifeSci Advisors, LLC
Brian Ritchie
Tel. : + 1 (212) 915-2578
Email: britchie@lifesciadvisors.com
FTI Consulting (for Ireland)
Jonathan Neilan or Patrick Berkery
Tel: +353 86 602 5988
Email: mainstay@fticonsulting.com
Mainstay Medical
Corporate Communications
Email: Media@mainstaymedical.com