- Centinel Spine® remains one of the fastest growing companies in the spine industry* and is dedicated exclusively to total disc replacement (TDR), one of the fastest growing segments in orthopedic implants.
- With the limited release of prodisc® C Nova, Centinel Spine now has four cervical total disc replacement (TDR) devices in clinical use in the U.S.
- Centinel Spine is the first and only company with a portfolio of cervical TDR devices that allows matching the disc to the needs of the patient and surgeon.
- prodisc is the most proven and extensively utilized TDR system in the world, offering both cervical and lumbar implants with anatomically-differentiated endplate designs.
WEST CHESTER, Pa., Oct. 31, 2024 /PRNewswire/ — Centinel Spine®, LLC (“the Company”), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced the first U.S. implantation of its prodisc C Nova cervical total disc replacement (TDR) product. In July 2022, the Company received U.S. Food and Drug Administration approval for 1-level indications for the prodisc C Nova device.
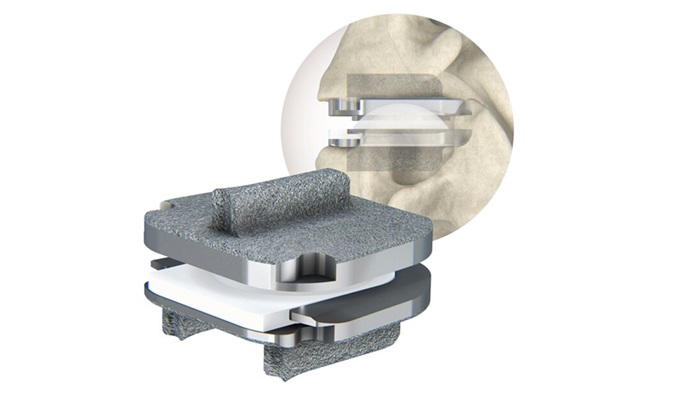
The prodisc C Nova technology is Centinel Spine’s fourth cervical TDR offering, and the third new cervical TDR product released in the U.S. by the Company since late 2022. The prodisc C Vivo and prodisc C SK products were both made available on a limited basis in late 2022 and have been used in over 8,000 procedures since release. Along with the originally available prodisc C cervical implant, which was launched in the U.S. in 2007, Centinel Spine has the broadest offering of cervical TDR solutions in the world to address surgeon preference and individual patient anatomy.
The first U.S. prodisc C Nova procedure was performed in Steamboat Springs, CO by orthopedic spine surgeon Dr. J. Alex Sielatycki of Steamboat Orthopedic & Spine Institute.
“The prodisc C Nova procedure went extremely well. The implant was easy to use and I believe it will serve this and many other patients very well,” said Dr. Sielatycki. “The tri-keel tracked nicely and allowed me to ensure good positioning of the implant. I also believe the initial fixation will be strong enough to allow this and other patients to return to full activity quickly. The prodisc C Nova implant will be a nice complement to the prodisc match-the-disc system, which provides the surgeon even more versatility when performing cervical disc replacement across the spectrum of patients with differing anatomy and pathology. I believe this patient and many more to come will benefit greatly from this technology,” Dr. Sielatycki adds.
prodisc C Nova was co-developed by spine surgeon Prof. Dr. Rudolf Bertagnoli, Chairman/CEO of Pro-Spine, Straubing, Germany. First implanted in Europe in 2009, the device features a unique tri-keel design and flat endplates for optimized implant positioning that allows surgeons to address individual patient anatomy. The low-profile keels enable a streamlined keel preparation technique and provide immediate fixation.
On this occasion, Prof. Dr. Rudolf Bertagnoli reflects, “prodisc C Nova has been my preferred cervical total disc replacement implant for many years. Its unique three-keel design, combined with the reliable prodisc motion mechanism, makes the prodisc C Nova particularly effective in my experience. The availability of a variety of anatomically-customized prodisc cervical endplates tailored to each patient offers substantial benefits for both surgeons and patients. With the introduction of the prodisc C Nova, these options are now further expanded for surgeons in the United States.”
Similar to all prodisc products, the prodisc C Nova device incorporates prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 250,000 implantations worldwide.**
Centinel Spine CEO Steve Murray adds, “We are delighted to make another proven prodisc cervical option available in the U.S. Our experience with prodisc C Vivo and prodisc C SK has shown that surgeons appreciate the variety of endplate designs for their consideration and selection in treating each patient. And, importantly, the entire prodisc portfolio is built on the proven, safe, and reliable motion preservation design that makes prodisc such a trusted name in spine care. We are the only spine company exclusively focused on motion preservation, and prodisc C Nova further amplifies this commitment.”
* Based on publicly available information.
** Data on file.
About Centinel Spine, LLC
Centinel Spine®, LLC is the leading global medical device company exclusively focused on addressing cervical and lumbar spinal disease with prodisc®, the most complete total disc replacement (TDR) technology platform in the world.
The Company’s prodisc technology is the most studied and clinically-proven TDR system across the globe, validated by over 540 published papers and more than 250,000 implantations. Centinel Spine’s prodisc is the only TDR technology with multiple motion-preserving anatomic solutions, allowing the surgeon to Match-the-Disc™ to each patient’s anatomy for both cervical and lumbar total disc replacement.
For more information, please visit the company’s website at www.CentinelSpine.com or contact:
Varun Gandhi
Chief Financial Officer
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: v.gandhi@centinelspine.com
SOURCE Centinel Spine, LLC