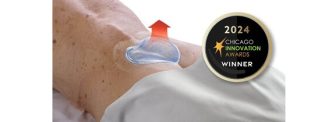
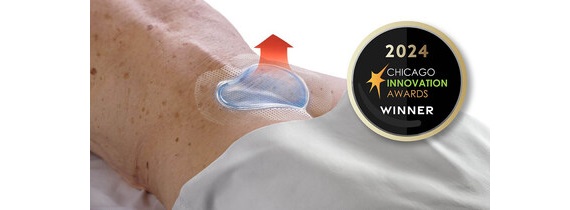
New collaboration to strengthen and deliver advanced soft tissue repair solutions for shoulder, foot and ankle procedures
WEST CHESTER, Penn. – November 14, 2024 – Johnson & Johnson MedTech, a global leader in orthopaedic technologies and solutions, announced today an exclusive commercial distribution agreement in the United States with Responsive Arthroscopy Inc., an innovative medical device company specializing in sports soft tissue repair solutions. This strategic agreement strengthens Johnson & Johnson MedTech’s sports platform, enhancing its soft tissue portfolio to meet the rising demand for advanced solutions in the rapidly growing sports soft tissue repair market.
“Our relationship with Responsive Arthroscopy is a significant step in expanding our leadership in orthopaedics and advancing our sports soft tissue offerings,” said Aldo Denti, Company Group Chairman, Orthopaedics, Johnson & Johnson MedTech. “This collaboration allows us to deliver a broader range of cutting-edge tools that empower surgeons in shoulder, foot and ankle procedures. We’re excited to drive value in this rapidly growing segment and remain committed to advancing patient care in meaningful ways.”
By combining Johnson & Johnson MedTech’s expertise in orthopaedic innovation with Responsive Arthroscopy’s advanced technologies, this agreement will provide healthcare providers with a more comprehensive set of tools to respond to evolving needs.
This collaboration marks a critical milestone in Johnson & Johnson MedTech’s mission to deliver innovative, impactful solutions that address evolving needs in orthopaedics and elevate patient outcomes.
Orthopaedic Solutions from Johnson & Johnson MedTech
Across Johnson & Johnson, we are tackling the world’s most complex and pervasive health challenges. In Orthopaedics, we are on a mission to keep people moving by leveraging our deep expertise in joint reconstruction, robotics and enabling tech, spine, sports, trauma, and extremities to develop the next generation of medtech solutions. We offer one of the most comprehensive Orthopaedics portfolios in the world that helps heal and restore movement for the millions of patients we serve. For more, visit our website or follow us at @jjmt_ortho and on LinkedIn.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more about our MedTech sector’s global scale and deep expertise in cardiovascular, orthopaedics, surgery and vision solutions at https://thenext.jnjmedtech.com. Follow us at @JNJMedTech and on LinkedIn. DePuy Synthes Products, Inc. is a Johnson & Johnson MedTech company.
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding the agreement with Responsive Arthroscopy Inc. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of DePuy Synthes Products, Inc., and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns of purchasers of health care products and services; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. NeitherDePuy Synthes Products, Inc., nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.