Geneva, November 19, 2024 – Spineart, a leader in spine surgery innovation, today announces that it has reached the significant milestone of 100,000 Ti-LIFE Technology cages sold worldwide, supporting the Company’s mission to transform spine surgery for surgeons, patients and hospitals in more than 60 global markets.
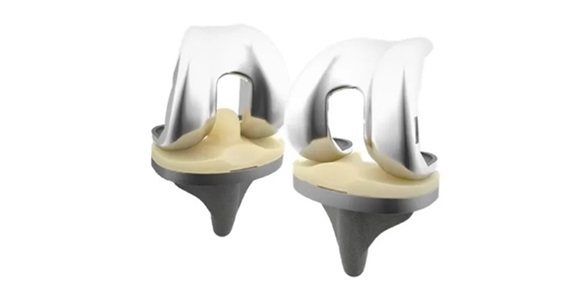
Launched in July 2016, Spineart’s Ti-LIFE Technology is based on a unique proprietary algorithm associated with a state-of-the art additive (3D printing) manufacturing process. Ti-LIFE is applied across a range of Spineart’s surgical implants, including posterior, lateral and cervical cages – hollow interbody devices used in spinal fusion surgeries. These cages act as a space holder between the vertebrae, helping to decompress the spinal cord and nerve root.
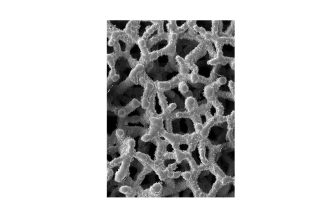
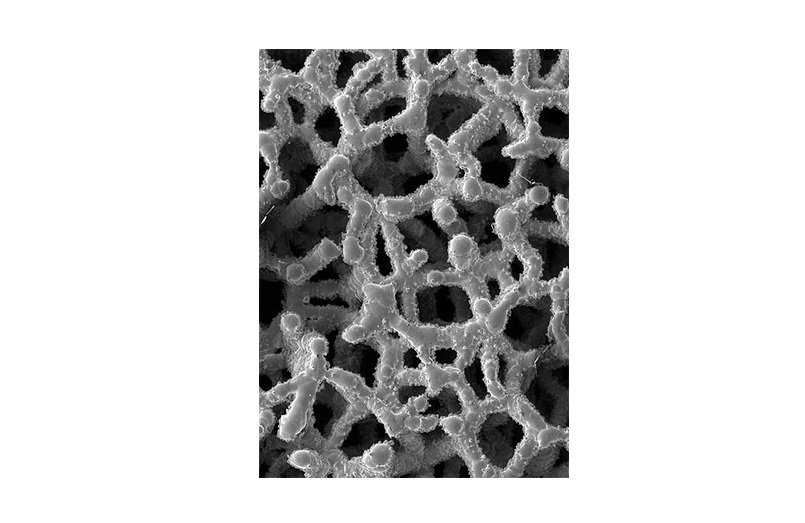
Ti-LIFE’s micro-porous structure closely mimics the architecture of the natural trabecular bone and is designed to promote osseous in-growth and cell colonization once implanted. Cages featuring the Ti-LIFE Technology have been shown to outperform other materials, demonstrating early and robust osseointegration without any bone graft or additive osteoinductive agents in just eight weeks.*
“Since its launch in 2016, Spineart’s Ti-Life Technology has contributed significantly to setting a new benchmark in spinal fusion surgery around the world,” remarked Jerome Trividic, Chief Executive Officer at Spineart. “As we progress towards the 20-year anniversary of the founding of our company, this latest milestone reflects our unwavering commitment to transforming spine surgery and improving patient lives, as well as Ti-LIFE’s excellent surgical track record.”
“Spineart’s Ti-LIFE Technology offers significant improvements over traditional titanium implants, with better osseointegration, customizable mechanical properties, and improved imaging capabilities, all of which contribute to enhanced patient outcomes in spinal fusion surgery,” said Joseph Laratta, MD, minimally invasive orthopedic spine surgeon at the Neck and Back Institute of Kentucky.
“Surpassing the 100,000 sales milestone for our Ti-LIFE Technology cages is a powerful demonstration of our strong growth to date as well as our future potential. Thank you to our team, investors, partners, surgeons and customers, each of whom have worked alongside us to reach this goal,” added Alessia Erlingher, Chief Commercial Officer at Spineart.
To learn more about Ti-LIFE Technology: https://www.spineart.com/technology/ti-life-technology/
References:
* Joseph L. Laratta, MD et al. 3D-printed titanium cages without bone graft outperform PEEK cages with autograft in an animal model. The Spine Journal. 2021; DOI.
About Spineart
Spineart is a global fast-growing company in spine surgery innovation, dedicated to accelerating the adoption of cutting-edge technologies for surgeons and hospitals worldwide for the benefit of their patients. Renowned for its commitment to Quality, Innovation, and Simplicity, Spineart continues to push the boundaries of spinal surgery with its comprehensive portfolio of procedural solutions and digital technologies. Spineart was awarded the “Prix de l’Economie Genevoise 2022” for its contribution to technological and scientific innovations, commercial activities, job creations and ESG principles.
Please visit www.spineart.com and follow on LinkedIn. For media inquiries or further information, contact press@spineart.com or visit www.spineart.com/media