AUSTIN, Texas, Jan. 14, 2025 /PRNewswire/ — DiFusion Technologies, an industry leader in advanced biomaterials for surgical implants, announced today the issuance of a new US Patent 12,178,936 B2 “Medical Implants and Methods of Manufacture.”
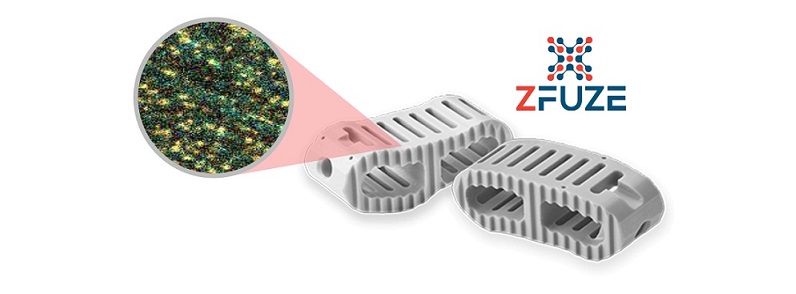
The new patent is encompasses four methods to manufacture ZFUZE® a proprietary therapeutic, anti-inflammatory, osseoconductive, load bearing biomaterial for orthopedic implants. ZFUZE is the first FDA-cleared advanced biomaterial for spinal implants that is proven to create a highly favorable cellular and immunologic response post-surgery. A recent impressive finding, is a 75 patient retrospective study, whereby ZFUZE posted a dominant 93.4% lumbar spinal fusion rate at 6 months while similar competitive studies demonstrate average lumbar fusion rates of 68% at 12 months.
“This new patent firmly protects our ZFUZE FDA Master File for another 17 years. ZFUZE is the only FDA cleared immuno-modulating biomaterial currently available to patients’ surgeons and hospitals,” DiFusion Founder and CEO Derrick Johns. “Building a FDA Master File is a long, arduous and expensive process that larger orthopedic companies are not scientifically equipped to execute,” added Johns.
About DiFusion Technologies:
DiFusion Technologies, Inc. is an industry leader in advanced biomaterials for surgical implants. DiFusion is revolutionizing the field of implant technology by developing innovative solutions that reduce the foreign body response from the patient’s immune system, thereby promoting early healing, reducing complications, and improving patient outcomes. The company’s proprietary manufacturing process produces implants that decrease chronic inflammation currently associated with mainstream biomaterials.
Contact: ceo@difusiontech.com (512) 574-1755 www.difusiontech.com
SOURCE DiFusion Inc.