RICHMOND, Va. and ATLANTA, Jan. 29, 2025 /PRNewswire/ — OrthoPreserve, a company developing orthopedic implant solutions, announced today it has been granted both a Breakthrough Device Designation and Total Product Life Cycle Advisory Program (TAP) enrollment from the U.S. Food & Drug Administration (FDA) for Defender, a meniscus replacement implant.
The designation covers the use of the therapeutic medical device to treat patients who continue to experience knee pain or impairment following a meniscus surgery, which impacts approximately 250,000 Americans annually. Prior to January 2025, orthopedic devices were not part of the TAP program, and OrthoPreserve’s device is believed to be the first Orthopedic device to win TAP enrollment.
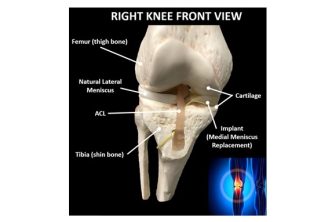
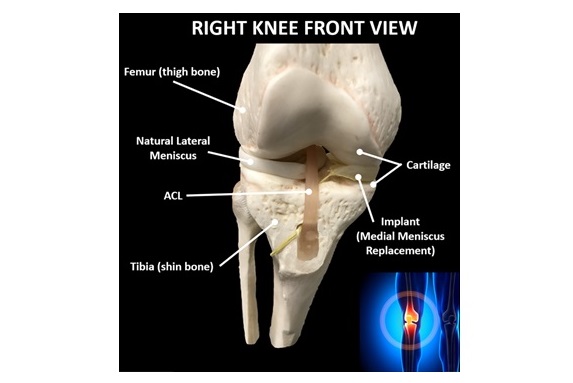
OrthoPreserve’s implant addresses a significant treatment gap that exists due to the poor outcomes of the standard of care for meniscus tears, a partial meniscectomy, which removes the damaged tissue. OrthoPreserve’s proprietary technology has the potential to disrupt the standard of care and displace more invasive treatments.
“While current meniscus surgeries and non-operative care can relieve pain temporarily, some patients do poorly in the intermediate to long term, progress to further surgeries, and develop degenerative arthritis that eventually requires a knee replacement. OrthoPreserve’s implant has the potential to offer a minimally invasive, durable solution and higher quality of life for patients suffering from pain and mobility issues after a meniscus tear,” said Kenneth Zaslav, M.D., Director of the Center for Regenerative Orthopedic Medicine at Northwell Lenox Hill Hospital and Professor of Orthopedic Surgery.
Through the Breakthrough Devices program and TAP enrollment, OrthoPreserve will have early and frequent communication with the FDA and non-FDA stakeholders, as well as priority review for future regulatory submissions. These programs are exclusively available for new medical devices that offer more effective treatment options for irreversibly debilitating conditions, and will allow for the company to accelerate development, assessment, and commercialization of Defender.
Brendan Baggot, Vice President of Regulatory Affairs for OrthoPreserve said, “TAP enrollment affords enhanced guidance by agency-selected advisors on a broad range of subjects that device startups struggle with, including market adoption and insurance coverage.”
“This Breakthrough Device designation from the FDA is a major milestone for OrthoPreserve and validates the longstanding unmet medical need that our meniscus implant is designed to address,” said Jonathan Schwartz, inventor, co-founder and CEO. “The anatomical design of the implant restores the normal stabilization and cushioning functions of the meniscus to relieve symptoms and preserve knee joint health.”
OrthoPreserve hopes to launch their Pilot clinical trial in 2026, while aiming for potential FDA approval by 2029.
About OrthoPreserve, Inc.
OrthoPreserve is a privately held medical device startup with technology spun out of Georgia Tech in 2021. The company is developing a new class of implant for knee meniscus replacement with a mission to restore mobility, reduce pain, and preserve quality of life for patients. OrthoPreserve has demonstrated feasibility of the implant in animal, cadaver, and benchtop studies with funding from the National Institutes of Health and private investors, and the company is planning human trials in the near future. The company is headquartered in Richmond, Virginia and has offices in Atlanta, Georgia.
The Defender implant is an investigational device and not available commercially.
Learn more at www.orthopreserve.com and follow us on LinkedIn
SOURCE OrthoPreserve, Inc.