- Innovation developed in partnership with the US company Omnia Medical
- Omnia launched PsiFGuard at the NANS convention in Orlando, from Jan. 30th to Feb. 1st, 2025
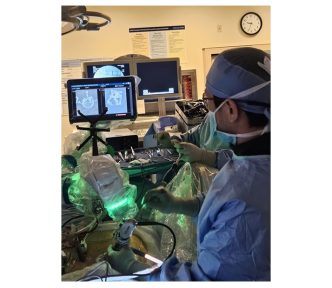
February 10, 2025 – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announces that its commercial partner Omnia Medical launched their codeveloped product PsiFGuard in the USA during the NANS (North American Neuromodulation Society) convention in Orlando, Florida (USA) from January 30th to February 1st, 2025.
Stéphane Bette, Deputy CEO and co-founder of SpineGuard, stated: “We are particularly excited to collaborate with Omnia Medical to launch PsiFGuard in the market with the support of the clinical experts from our team who recently joined Omnia. PsiFGuard is part of an innovative solution combining our technologies into an integrated kit matching perfectly the practical needs of healthcare providers offering ambulatory sacro-iliac joint fusion procedures to patients. Omnia Medical is the pioneer company in this area and continues to deliver to physicians an unmatched clinical value proposition thanks to the efficacy of our PsiFGuard device in locating the SI joint. For SpineGuard, this new sacroiliac application of our DSG® technology embedded in a well reimbursed fast-growing procedure represents a game-changing opportunity of fast adoption by the market and significant contribution to the growth of our revenue.”
Troy Schifano, CEO and Co-Founder of Omnia Medical, added: “The NANS convention served to strengthen our conviction that PsiFGuard is an enabling technology needed by the market to bolster physicians’ confidence that they are properly placing the implant in the sacroiliac joint for maximum efficacy. The convention was well attended, and many physicians are eager to be trained on our procedure with this technology. When coupled with the results and feedback from the procedures we did prior to NANS, we expect solid growth through the year and beyond from a rapidly expanding base of physicians.”
2025 Outlook
In 2025, SpineGuard continues to focus on sustaining its sales momentum, by relying on the introduction of its new products on the market, the Threaded PediGuard for scoliosis correction via anterior approach recently CE marked, and the PsiFGuard device co-developed with Omnia Medical for sacroiliac fusion which recently obtained FDA clearance. The Company is also pursuing the registering process of the whole PediGuard product range in China. Moreover, SpineGuard is working on building strategic partnerships and on strengthening its financial position in continuing to explore various financing options. To date, the Board of Directors has adopted the going-concern principle based on the Company’s consolidated cash position, cash equivalents, forecast inflows, ongoing commercial growth, the recent signature of a bond contract for a maximum amount of €1 million and the measures implemented by the management to secure the Company’s financing.
About SpineGuard®
Founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard is an innovative company deploying its proprietary radiation-free real time sensing technology DSG® (Dynamic Surgical Guidance) to secure and streamline the placement of implants in the skeleton. SpineGuard designs, develops and markets medical devices embedding its technology. Over 100,000 surgical procedures have been secured worldwide thanks to DSG® and 34 studies published in peer-reviewed scientific journals have demonstrated the multiple benefits DSG® offers to patients, surgeons, surgical staff and hospitals. Building on these strong fundamentals and several strategic partnerships, SpineGuard is expanding the scope of its DSG® technology to the treatment of scoliosis via anterior approach, sacroiliac joint fusion, dental implantology and innovations such as the « smart » pedicle screw and power drill or surgical robotics. DSG® was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer. SpineGuard has engaged in multiple ESG initiatives.
For further information, visit www.spineguard.com
About Omnia Medical
Top medical device engineers and industry professionals came together in 2014 to form Omnia Medical, an orthopedic implant company located in Morgantown, WV. Omnia Medical’s mission is to develop novel products that reduce operative time through safe and reproducible instrumentation, while achieving superior surgical outcomes. Ongoing surgeon collaboration helps the company achieve this mission, which leads to critical cost savings for healthcare providers and their patients. Omnia Medical has created a comprehensive range of spine implants, including a proprietary line of interbodies with TiBrid osseointegrative surface technology, and is notable for being among the pioneers in posterior sacroiliac joint (SIJ) fusion. For more information, visit www.OmniaMedical.com.
Disclaimer
The SpineGuard securities may not be offered or sold in the United States as they have not been and will not be registered under the Securities Act or any United States state securities laws, and SpineGuard does not intend to make a public offer of its securities in the United States. This is an announcement and not a prospectus, and the information contained herein does and shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities referred to herein in the United States in which such offer, solicitation or sale would be unlawful prior to registration or exemption from registration.
Contacts
SpineGuard
Pierre Jérôme
CEO & Chairman
Tel: +33 1 45 18 45 19
p.jerome@spineguard.com
SpineGuard
Anne-Charlotte Millard
CFO
Tél. : 01 45 18 45 19
ac.millard@spineguard.com
NewCap
Investor Relations & Financial Communication
Mathilde Bohin / Aurélie Manavarere
Tel.: +33 1 44 71 94 94
spineguard@newcap.eu