Building on last year’s momentum of 18 FDA 510(k) Clearances and 45 global product launches, the company accelerates innovation across joint reconstruction, trauma, spine, and more
PALM BEACH GARDENS, Fla., March 10, 2025 /PRNewswire/ — Johnson & Johnson MedTech, a global leader in orthopaedic technologies and solutions, is highlighting its latest advancements in digital orthopaedics at the American Academy of Orthopaedic Surgeons (AAOS) 2025 Annual Meeting in San Diego, California, from March 10-14.
Expanding on last year’s innovations, Johnson & Johnson MedTech is introducing cutting-edge implants, advanced techniques, and data-driven technologies across orthopaedic specialties, including joint reconstruction, trauma, extremities, and spine. These developments are grounded in the company’s commitment to deliver innovative, impactful solutions that address the evolving needs of surgeons and patients.
“With over 1.7 billion people worldwide living with musculoskeletal conditions—more than cancer, heart, and lung diseases combined—the need for effective, scalable orthopaedic solutions has never been greater. At AAOS 2025, we are excited to showcase how our innovations are transforming the full ecosystem of orthopaedic procedures to meet this growing demand,” said Aldo Denti, Company Group Chairman, Orthopaedics, Johnson & Johnson MedTech. “By seamlessly integrating innovative implants, advanced surgical techniques, and data-driven enabling technologies, we’re helping surgeons advance patient care while improving efficiency for hospitals and ambulatory surgery centers.”
For over 135 years, the company has been at the forefront of orthopaedic innovation, beginning with the world’s first orthopaedic device—a fitted splint for setting broken bones. Today, it continues this legacy by delivering digitally connected solutions that empower surgeons with enhanced capabilities in precision and efficiency.
Key innovations on display at AAOS 2025 include:
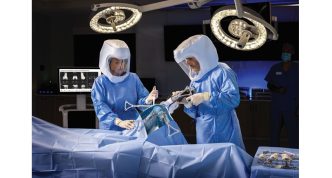
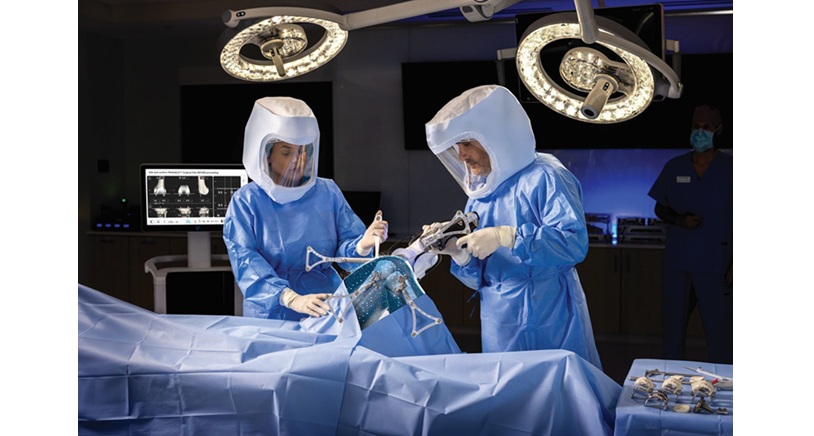
- VELYS™ Robotic-Assisted Solution (VELYS™): With more than 100,000 total knee replacement procedures performed across 31 global markets1, this CT-free digital system2 is redefining total knee replacements by helping surgeons improve precision, streamline workflows,3,4 and provide more personalized care.3,5 The kinematically designed6-8 and clinically proven9-11 ATTUNE™ Knee System integrates seamlessly with VELYS™, reducing the risk of revision by 33% compared to similar total knee replacements.12
Recently, the U.S. Food and Drug Administration (FDA) cleared VELYS™ for unicompartmental knee arthroplasty (UKA) in both medial and lateral procedures. VELYS™ helps address alignment and precision challenges faced in manual partial knee replacement by enabling surgeons to guide precise implant placement without a CT scan. 1,4,13-15*† The SIGMA™ HP implant is compatible with VELYS™ and demonstrates improved 14-year survivorship compared to similar implants, as shown in the national joint registry.16
- KINCISE™ 2 Surgical Automated System: Hip and knee procedures require repetitive and forceful motions, placing significant physical demands on surgeons.17 Designed with surgeon comfort in mind, this next-generation power tool features automation technology, customizable options and new capabilities like acetabular cup extraction – all developed to reduce the physical demands on surgeons during hip or knee procedures.18-22‡
- VELYS™ Active Robotic Assistance Solution: Each year, over 4.83 million spinal surgeries are conducted worldwide, with 1.34 million occurring in the United States alone.23 Despite the prevalence of these procedures, first-generation robotic systems can pose significant challenges for surgeons. In response, Johnson & Johnson MedTech developed a groundbreaking technology in collaboration with top spine surgeons, specifically aimed at addressing the complexities of advanced spine procedures – the VELYS™ Spine system. This innovative platform integrates standalone navigation with next-generation active robotic technology, providing flexibility to tailor surgical workflow.
- VOLT™ Trauma System Expansion: VOLT™ (Variable Angle Optimized Locking Technology) Plating System combines dynamic compression with the flexibility of variable angle locking without compromising stability.24-27§ VOLT™ has obtained European CE Marking¶, following FDA 510(k) clearance and commercial launch in the United States. VOLT™ will launch in select European markets in 2025.
- INHANCE™ INTACT™ for Shoulder Replacement: Recently 510(k) cleared by the FDA, this proprietary instrument set is designed for use with the INHANCE™ Shoulder System in subscapularis-sparing (SCS) total anatomic shoulder replacements. Patients are able to move their shoulder without restrictions from the first day post-operatively in subscapularis-sparing approach compared to 6 weeks of immobilization with the traditional approach.28 When combined with TRUMATCH™ 3D pre-surgical planning cloud system,# surgeons can tailor SCS procedures to each patient’s unique anatomy for a more personalized approach.28
- Advance Case Management: Powered by AI, the Advance Case Management system is a digitally integrated solution that simplifies pre-surgery processes for ambulatory surgery centers (ASCs) and outpatient centers by utilizing case schedules and patient data. This translates to greater operating room efficiency through reduced turnover times,29 smaller inventory footprints, lower sterilization costs,30 and a better patient experience.
- Wound Closure and Healing in Orthopaedic Surgery: The combined use of STRATAFIX™ and DERMABOND™ PRINEO™ System may offer both surgeon and patient benefits in total joint arthroplasty.†† Wound closure with STRATAFIX™ is faster and more resource efficient than standard-of-care wound closure in total joint arthroplasty. Additionally, the patient group using STRATAFIX™ and DERMABOND™ PRINEO™ reported significantly higher patient satisfaction and cosmetic outcomes.32-34**
For more information on the Orthopaedics and Surgery portfolios, visit Johnson & Johnson MedTech at AAOS 2025 in Booth #4329 or learn more online.
Orthopaedic Solutions from Johnson & Johnson MedTech
Across Johnson & Johnson, we are tackling the world’s most complex and pervasive health challenges. In Orthopaedics, we are on a mission to keep people moving by leveraging our deep expertise in joint reconstruction, robotics and enabling tech, spine, sports, trauma, and extremities, to develop the next generation of medtech solutions. We offer one of the most comprehensive Orthopaedics portfolios in the world that helps heal and restore movement for the millions of patients we serve. For more, visit our website or follow us at @jjmt_ortho and on LinkedIn.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow and profoundly impact health for humanity. Learn more about our MedTech sector’s global scale and deep expertise in cardiovascular, orthopaedics, surgery and vision solutions at https://thenext.jnjmedtech.com. Follow us at @JNJMedTech and on LinkedIn. DePuy Synthes Products, Inc. is a Johnson & Johnson company.
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding the VELYS™ Robotic-Assisted Knee Solutions, ATTUNE™ Knee System, SIGMA™ HP implant, KINCISE™ 2 Surgical Automation System, VELYS™ SPINE, VOLT™ Plating System, INHANCE™ INTACT™ Shoulder Tissue Sparing Technique, Advanced Case Management system, STRATAFIX™ Knotless Tissue Control Devices, and DERMABOND™ PRINEO™ Skin Closure System. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of DePuy Synthes Products, Inc. and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns of purchasers of health care products and services; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s most recent Annual Report on Form 10-K, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Neither DePuy Synthes Products, Inc. nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
*As compared to manual/conventional
†Based on a survey of 21 surgeons who used the system in a cadaveric procedure.
‡Compared to KINCISE™ V1
§Cantilever testing done comparing VOLT™ Mini and Small Fragment Systems to VA LCP™ Plating System, and LCP™ Plating System
¶Only for VOLT™ Mini Fragment and VOLT™ Small Fragment Plating Systems.
#Manufactured by Materialise NV
|| Based on a market research online survey of 152 orthopedic surgeons who rated their agreement with each statement. Percentages are calculated as percentage of respondents who answered “4- agree somewhat” or “5- agree strongly” on a 5-point Likert scale.
††As shown in 3 prospective randomized trials: 60 TKAs comparing STRATAFIX™ to interrupted suture in arthrotomy closure, 60 THAs comparing STRATAFIX™ to interrupted suture in arthrotomy closure, and 60 TKAs comparing STRATAFIX™ to staples in the subcuticular layer and comparing DERMABOND™ PRINEO™ to staples in skin closure.
**In a prospective randomized trial of 60 TKAs comparing STRATAFIX™ to staples in the subcuticular layer and comparing DERMABOND™ PRINEO™ to staples in skin closure. In both groups, the arthrotomy and subcutaneous layers were closed with braided suture.
The third-party trademarks used herein are the trademarks of their respective owners.
Important Information: Prior to use, refer to the instructions for use supplied with the device(s) or indications, contraindications, warnings and precautions.
© Johnson & Johnson and its affiliates 2025. All rights reserved. US_ORT_ALLO_396594
Media Contacts:
Lindsey Diaz-MacInnis
ldiazmac@its.jnj.com
Abhi Basu
abasu26@its.jnj.com
- DePuy Synthes. WW VRAS Array Set Sales. 2024
- Premier HOPD 2018-2020 Q1 (December 2020). Internal Report.
- Doan, G.W., et al., Image-Free Robotic-Assisted Total Knee Arthroplasty Improves Implant Alignment Accuracy: A Cadaveric Study. J Arthroplasty, 2022. 37(4): p. 795-801.
- DePuy Synthes User Experience Evaluation of the VELYS Robotic-Assisted Solution for Total Knee. July 2020.Windchill #103744839
- Clatworthy M. Patient-Specific TKA with the VELYS Robotic-Assisted Solution. Surg Technol Int. 2022;40
- Mane AM, Komistek RD, Heldreth MA, “The soft tissue harmony with the knee motion in Cruciate Retaining Arthroplasty”, ORS-2021
- Khasian M, Sharma A, Fehring TK, Griffin WL, Mason JB, Komistek RD. Kinematic Performance of Gradually Variable Radius Posterior-Stabilized Primary TKA During Various Activities: An In Vivo Study Using Fluoroscopy. J Arthroplasty. 2020;35(4):1101-8.
- List R, Schütz P, Angst M, Ellenberger L, Dätwyler K, Ferguson SJ. Videofluoroscopic Evaluation of the Influence of a Gradually Reducing Femoral Radius on Joint Kinematics During Daily Activities in Total Knee Arthroplasty. J Arthroplasty.
- Meermans G, Galvain T, Wigham R, Do Rego B, Schroer D. Comparative analysis investigating the impact of implant design on hospital length of stay and discharge destination in a Dutch hospital with an established enhanced recovery program. J Arthroplasty. 2020;35(1):182-187.
- Etter K, Lerner J, Kalsekar I, de Moor C, Yoo A, Swank M. Comparative analysis of hospital length of stay and discharge status of two contemporary primary total knee systems. J Knee Surg. 2018;31(6):541-550.
- Bruggenjurgen B, Muehlendyck C, Gador LV, Katzer A. Length of stay after introduction of a new total knee arthroplasty (TKA)-results of a German retrospective database analysis. Med Devices (Auckl). 2019;12:245-251.
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR), Ad Hoc Report, ID No.4067 for DePuy Synthes, ATTUNE Total Knee (Procedures from 16 Aug 1999 – 25 Sep 2024), Report Approved 2nd Oct 2024, AOA, Adelaide: 1-27.
- DePuy Synthes VELYS™ Robotic-Assisted Solution for Unicompartmental Knee Rev-C Surgical Technique Guide. January 2024. Windchill #501433682
- DePuy Synthes Rev A VELYS Robotic-Assisted Solution for Unicompartmental Knee User Guide. January 2024. IFU #501488496
- Doan et al. “Assessment of Accuracy of a Novel Image Free Robotic-Assisted System for Unicondylar Knee Arthroplasty.” World Arthroplasty Congress, Poster No. 282, April 2024.
- National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Implant summary report for DePuy Sigma HP (Uni) Unicondylar. NJR Database extracted 31 July 2024, pages 4-6. Licensed for use until 16 Aug 2025
- Ferrari E, et al. Feasibility of using Electrocardiography (ECG) data to quantify surgeon’s burden within manual and automated THA: results from a prospective randomized controlled trial. Poster presented at: Orthopaedic Research Society; February 10-14 2023; Dallas, TX
- Johnson & Johnson MedTech. KINCISE System Acetabular Cup Extraction – Instructions For Use. 2023. Windchill 502101159.
- Johnson & Johnson MedTech. KINCISE V1 – KINCISE V2 Comparison Report. 2024. Windchill 502047070.
- Johnson & Johnson MedTech. KINCISE V2 – Performance and Lifetime. 19/08/24. Windchill 501439497
- Johnson & Johnson MedTech. KINCISE 2 Surgical Automated System – Instructions For Use. 2024. Agile SE_933224.
- Johnson & Johnson MedTech. KINCISE V1 Performance and Lifetime Verification. 12/12/19. Windchill 0000283683.
- D’Souza M, et al. Robotic-assisted spine surgery: history, efficacy, cost, and future trends. Robot Surg. 2019;6:9-23.
- DePuy Synthes VOLT 2.0 – Marketing Claims Interface Testing. September 4, 2024. Windchill # 501969571
- DePuy Synthes VOLT 2.4 – Marketing Claims Interface Testing. September 4, 2024. Windchill # 502025403
- DePuy Synthes VOLT 2.7 – Marketing Claims Interface Testing. September 4, 2024. Windchill # 502025404
- DePuy Synthes VOLT 3.5 – Marketing Claims Interface Testing. September 2, 2024. Windchill #501969506
- Lee S, Sardar H, Horner NS, Al Mana L, Miller BS, Khan M, et al. Subscapularissparing approaches in shoulder arthroplasty: a systematic review. J Orthop 2021; 24:165–72.
- Novant Health Project Report Deck. Johnson and Johnson Medical Device Companies; 2017.
- Capra R, et al. Implementing a perioperative efficiency initiative for orthopedic surgery instrumentation at an academic center: A comparative before-and-after study. Medicine (Baltimore). 2019;98(7):e14338.
- Buzzback (2025). Orthopedic Experiential Marketing Research.
- Sundaram K, Piuzzi NS, Patterson BM, et al. Skin closure with 2‑octyl cyanoacrylate and polyester mesh after primary total knee arthroplasty offers superior cosmetic outcomes and patient satisfaction compared to staples: a prospective trial. Eur J Orthop Surg Traumatol. 2019; 10.1007_s00590-019-02591-4.
- Sundaram K, Piuzzi NS, Klika AK, et al. Barbed sutures reduce arthrotomy closure duration and suture utilization compared to interrupted conventional sutures for primary total hip arthroplasty: a randomized controlled trial. Hip Int. 2020 Mar 19:1120700020911891. doi: 10.1177_1120700020911891. Epub ahead of print. PMID: 32188284.
- Sundaram K, Warren JA, Klika A, et al. Barbed sutures reduce arthrotomy closure duration compared to interrupted conventional sutures for total knee arthroplasty: a randomized controlled trial. Musculoskelet Surg. 2020 Mar 7. doi: 10.1007_s12306-020-00654-y. Epub ahead of print. PMID: 32146687.
SOURCE Johnson & Johnson MedTech