SEATTLE, WA and MONTREAL, QC – May 20, 2025 / OrthoSpineNews / – In a groundbreaking advancement for the orthopedic device industry, Sawbones and Numalogics, Inc. are proud to announce that their jointly developed Finite Element Analysis (FEA) model for orthopedic screw pullout testing has received formal qualification from the United States Food and Drug Administration (FDA) through its Medical Device Development Tools (MDDT) program¹. This achievement marks the first-ever FDA qualification of a mechanical test for an orthopedic device, setting a new precedent for virtual testing in regulatory science.
The qualified tool simulates screw pullout behavior in accordance with ASTM F543², a widely accepted standard for evaluating the mechanical performance of orthopedic screws. Built on Sawbones’ industry-standard synthetic bone materials and powered by Numalogics’ advanced FEA modeling technology, the model enables accurate, reproducible virtual testing, reducing reliance on time-consuming and costly physical bench tests. This validated virtual method allows orthopedic device manufacturers to replace or supplement physical testing for both product design optimization and regulatory submissions.
“This landmark FDA qualification highlights and elevates the role of standardized bone simulation in medical device development and regulatory science,” said Amy Posch, M.S. Design Engineer-Biomechanical at Sawbones®, “Together with Numalogics, we’ve delivered a tool that is both scientifically rigorous and regulatory-ready.”
By qualifying this virtual test, the FDA has recognized its ability to produce trustworthy and standardized evidence as a surrogate to physical testing using ASTM F543 screw pullout testing protocols, and synthetic bone materials manufactured per ASTM F1839³.
“This isn’t just a technical validation — it’s a shift in how the orthopedic industry can approach design and regulatory submissions,” said Eric Gaudreau, President of Numalogics. “With this FDA-qualified tool, companies can now leverage digital tools to reduce risk, accelerate development, and improve consistency.”
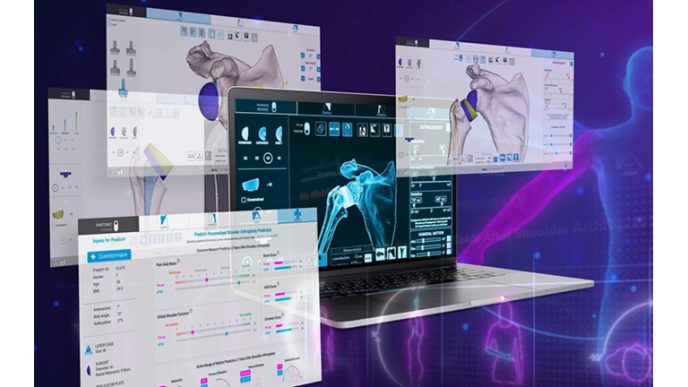
Interested users can access the tool directly through the Sawbones’ ENDPOINT™ platform, a suite of virtual orthopedic implant test tools, at: https://www.sawbones.com/endpoint-virtual-orthopedic-mechanical-implant-tests-fea
Footnotes:
¹ Medical Device Development Tools (MDDT) Program – An FDA initiative that qualifies tools (such as models, clinical trial simulations, or biomarker tests) for use in medical device development and regulatory review, ensuring they meet standards for scientific validity and reliability in a specific context of use. Learn more
² ASTM F543 – Standard specification from ASTM International for metallic medical bone screws, including test methods for evaluating properties such as pullout strength and torque.
³ ASTM F1839 – Standard specification for rigid polyurethane foam intended as a standardized material for use in testing orthopedic devices, ensuring consistency in mechanical testing protocols.
About Sawbones®
In addition to supplying the world’s best medical procedure simulation models, Sawbones® offers a complete range of composite bones and test materials for orthopaedic experimental and computer simulated biomechanics. Designed to simulate the physical properties of human bone; these materials offer a more reliable test bed for biomechanical studies than cadaveric specimens. As an orthopaedic research community, Sawbones® has been qualifying and using biomechanical test materials for over 30 years with their use becoming more prevalent in scientific journals. Sawbones® are an active member of ASTM and ISO subcommittees for medical devices and implants for surgery.
About Numalogics Inc.
Numalogics specializes in computer modeling and simulation for the medical device, sports equipment, and military industries. In addition to providing consulting services that can help solve product development and innovation challenges, Numalogics is carving a path to develop easy-to-use software applications that would allow product innovators to perform simulation testing, without requiring the dedicated skill and experience in computer simulation. To ensure models are verified and validated, Numalogics is an active contributor to the ASME V&V 40 Committee.
Media Contacts:
Sawbones
Cheyanne Webster
Marketing Manager
cheyanne@pacific-research.com
Numalogics
Dustin Arless
Director, Business Development
darless@numalogics.com