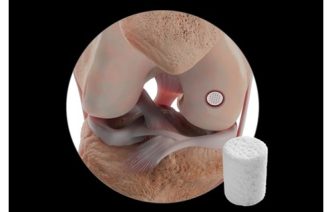
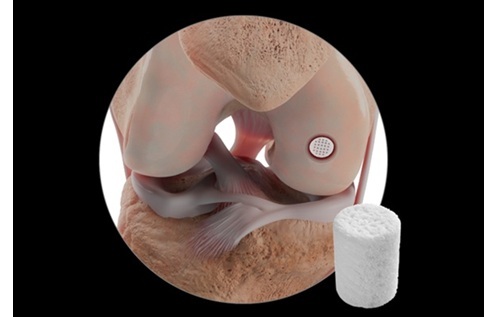
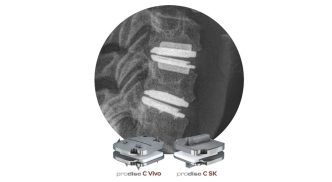
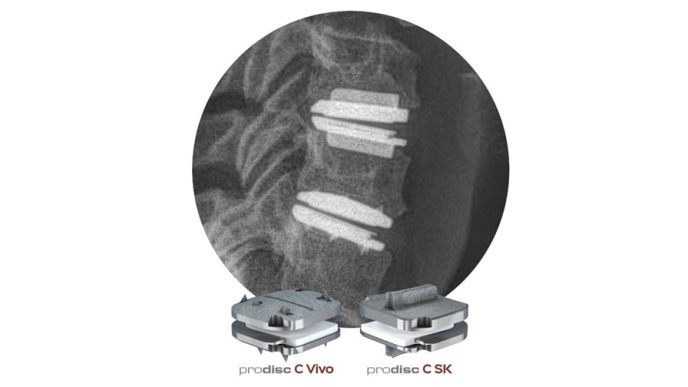
Janet Trunzo, AdvaMed’s senior executive vice president of technology and regulatory affairs, moderates a discussion with leaders of the Center for Devices and Radiological Health on Wednesday, Oct. 8, 2025. Trunzo, who hosted the panel one last time before her retirement, was the only person stage on this year as CDRH leaders attended remotely. Elise Reuter/MedTech Dive, data from MedTech Dive
Michelle Tarver, director of the Center for Devices and Radiological Health, and other center officials attended virtually as a government shutdown continues.
By Elise Reuter, Senior Reporter –
SAN DIEGO, October 9, 2025 — At AdvaMed’s annual conference, top medical device regulators typically have a significant presence. Food and Drug Administration officials speak on panels and chat with industry representatives, culminating in an ending keynote where leaders from the Center for Devices and Radiological Health take audience questions.
This year, there were plenty of questions to ask, as the medtech industry prepares for a new round of user fee negotiations, looks to understand the impact of FDA staff cuts, grapples with the possibility of increased tariffs and tries to pin down the Trump administration’s policy priorities.
Amid the backdrop of a U.S. government shutdown that has extended for more than a week, the FDA had a much smaller presence at the event. Top CDRH leaders, including director Michelle Tarver, tuned in virtually for Wednesday’s town hall, but no audience questions were taken. Janet Trunzo, AdvaMed’s senior executive vice president of technology and regulatory affairs, asked prepared questions to CDRH leaders from onstage.
Tarver answered a question about how her first year leading the CDRH has been, as she was named director shortly after speaking at last year’s conference.
“I think a roller coaster is probably the best word to explain this first year,” Tarver said. “There’s definitely many unexpected changes.”