HUNTSVILLE, Ala., January 21, 2026 /OrthoSpineNews/ – Curiteva, Inc., a privately held, technology and manufacturing company, is proud to announce the significant achievement of over 10,000 levels successfully treated using the Inspire implant portfolio. With 5,000 interbodies implanted in just the last several months, this milestone underscores the rapid market adoption and transformative impact of the company’s novel 3D-printed Trabecular PEEK with HAFUSE technology. This achievement coincides with the alpha launch of the Inspire Standalone ALIF system and the FDA clearance for the nano technology designation of Inspire 3D Printed Trabecular PEEK Technology, further enhancing and expanding the portfolio.
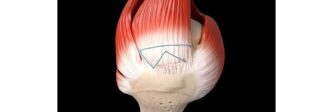
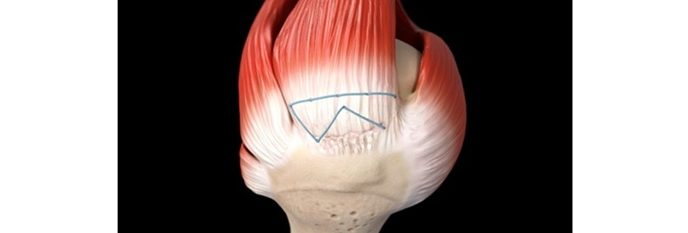
Jeffrey Mullin, MD, from University of Buffalo Neurosurgery shared his experience, stating “After using the Inspire Technology for the past two years in my high-risk patients, I’ve seen firsthand the rapid bone growth through and around the fusion construct, all without seeing any cases of pseudarthrosis or cage subsidence. This milestone is not only a celebration of the patients’ lives impacted but, it’s likely just the beginning of rapid market adoption given the exceptional outcomes when using the Inspire technology”.
- Since launch in April of 2023 more than 10,000 interbodies have been implanted
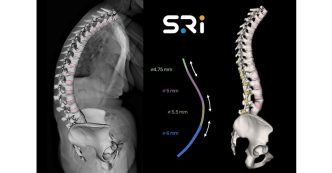
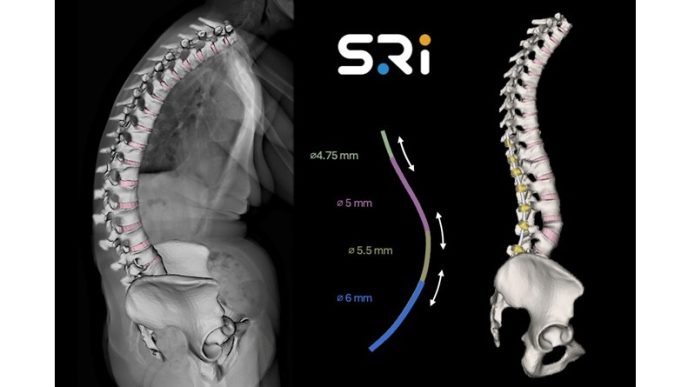
- The Inspire line is now comprised of Cervical, Posterior, and Anterior Lumbar Portfolios
- Inspire has resolved PEEK’s shortcomings by creating a hydrophilic, bioactive environment for cell attachment, proliferation, and healing
- Inspire is featured in The Journal of Biomaterials with two more publications anticipated this year
- Curiteva doubled printer capacity in 2025 to meet growing demand
“Dr. Mullin’s experience echoes what we consistently hear from physicians using Inspire – incredible results with minimal-to-no adverse events, even in the most challenging patients.” Commented David Schmidt, Curiteva’s Chief Commercial Officer. “With an impressive 60% growth in surgeries and a 90% increase in our surgeon customer base over the past year, Inspires momentum shows no signs of slowing down. 2026 is poised to be a breakout year for Inspire and Curiteva.”
Curiteva plans to expand the application of the Inspire Technology into further surgical approaches, with standalone cervical and lateral systems currently prioritized for development.
About Curiteva:
Curiteva is a privately held technology and manufacturing company based in Huntsville, Alabama. Our business is founded on a commitment to building world-class manufacturing, accelerating research and development, maintaining lean operational discipline, and delivering novel technology to meet the evolving needs of our customers and the patients they serve. Curiteva is pioneering 3D printing of Trabecular PEEK implants with a bioactive nano-surface to revolutionize how engineered structures and implant biomaterials accelerate immunomodulation, enhance healing, and improve patient outcomes. For more information, please visit www.curiteva.com