MORRISVILLE, N.C., Sept. 17, 2024 /PRNewswire/ — Sparta Biomedical, a private medical device company focused on osteoarthritis repair solutions, today announced that it has successfully implanted its Ormi device in patients with focal knee lesions as part of its first-in-human clinical trial. The ongoing prospective, multi-center, single-arm study is being conducted at sites in the Dominican Republic and Colombia, where patients have already been enrolled and treated.
The first-in-human study is focused on assessing the safety of Ormi in patients experiencing knee pain due to the loss of articular cartilage in the femoral condyle. The device’s unique design allows surgeons to address cartilage damage with or without involvement of the underlying bone. Following implantation, study investigators will monitor patients closely using imaging, physical exams, and patient-reported outcome measures.
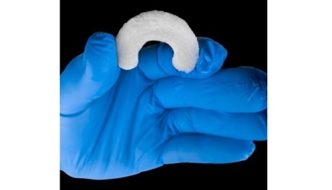
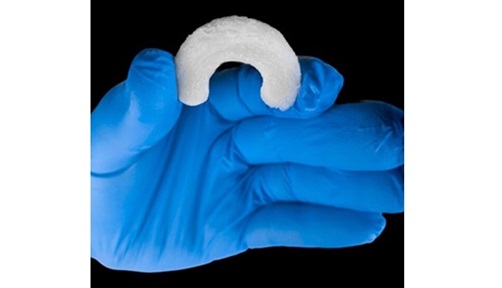
The Ormi device leverages Sparta Biomedical’s Galene platform, a novel synthetic cartilage that closely replicates the properties of native hyaline cartilage. Ormi integrates Galene with a proprietary titanium stemmed base to ensure secure fixation during the repair of cartilage lesions. Ormi aims to address the limitations of current conservative and surgical treatments for knee osteoarthritis, offering a potential solution that supports weight-bearing and full range of motion while minimizing pain within a short timeframe.
“Helping patients with knee focal lesions restore mobility and reduce pain quickly has been a long-standing issue. Our goal is to provide orthopedic surgeons with a simple, highly effective solution that is not dependent on patient biology. No approved device today has Ormi’s features. The reality is focal lesions in the knee affect the lives of hundreds of millions worldwide, and surgeons, depending on geography, have few to no solutions to treat their patients. We are excited to be one step closer to showcasing Ormi’s clinical benefits and helping clinicians get their patients feeling better soon,” said Dushyanth Surakanti, Co-Founder & CEO.
Active study sites include Clinica de la Mujer in Bogota, Colombia, and HOMS in Santiago de los Caballeros, Dominican Republic. Dr. Andres Garcia Martinez, Investigator and Head of Orthopedic Surgery at HOMS, shared, “My colleagues and I are excited to see how well patient recovery has been,” Dr. Andrés García-Núñez, Investigator, stated, “I am very encouraged by what I am seeing so far.” Dr. Jeremen Silva, Investigator at Clinica de la Mujer said “Ormi has the potential to address the needs of so many of my patients who otherwise would not be treated. I am equally impressed with the ease of implantation and robust fixation.”
About Sparta Biomedical:
Sparta has developed a first-of-its-kind device, Ormi™, to treat knee osteoarthritis. The US FDA granted Ormi Breakthrough Device Designation.
The impact of knee osteoarthritis cannot be ignored, with a staggering 651 million people worldwide affected by this debilitating condition. The associated morbidity and cost of this disease are significant, making it crucial to find effective solutions. Ormi is unlike other technologies that focus on cartilage regeneration approaches, which can take a long time to grow and are not as strong as the original, Ormi mimics the properties of healthy human cartilage right from day one, providing an innovative and groundbreaking solution to this widespread problem.
For more information, please visit https://www.spartabiomedical.com/
Ormi™ is not approved by the FDA and not commercially available. It is in development. Approvals and clearances are subject to testing, results, and the FDA review process.
SOURCE Sparta Biomedical