OrthoSpineNews – March 6, 2025 – In a major step forward for orthopedic innovation, the FDA has approved the compassionate use of Hip Innovation Technology’s (HIT) Reverse Hip Replacement System (Reverse HRS) for a patient with spinal fusion. This decision not only underscores the growing need for specialized solutions in hip arthroplasty and represents a potential breakthrough for high-risk hip replacement patients.
For years, patients with spinal fusion and spinal pelvic disorders have faced an elevated risk of hip replacement complications—particularly dislocation. Traditional hip replacement systems were not designed with their altered biomechanics in mind, leading to significantly higher failure rates. The research speaks for itself: studies have shown an 80% increase in hip dislocations within six months for patients with lumbar fusion, as well as an increased risk of revision surgeries. Yet, until now, little had been done to develop an implant addressing this unique challenge.
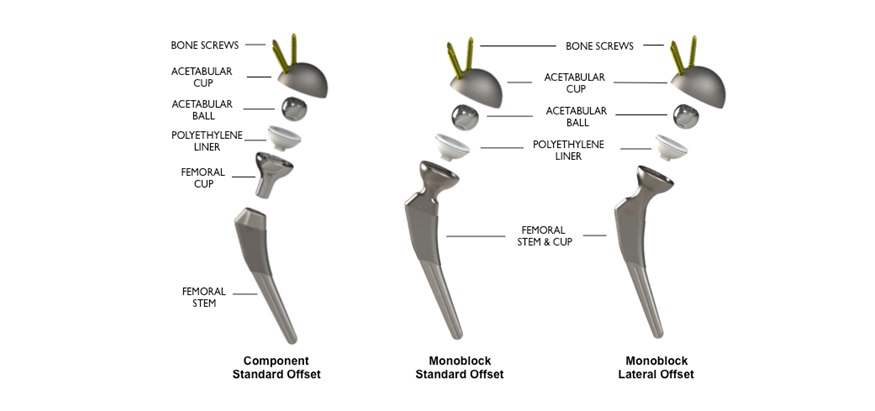
Enter the Reverse HRS. With its reverse geometry design, the implant aims to improve hip stability, allow for greater ranges of motion, and reduce the risk of dislocation. By shifting the ball component into the acetabular cup rather than the femoral stem, the system preserves the natural center of rotation while introducing a more forgiving design to compensate for suboptimal component positioning. The result? A potential game-changer for high-risk patients.
“We are extremely encouraged by the opportunity to offer the Reverse Hip Replacement System to an at-risk patient,” said Stephen J. Zabinski, MD, Medical Director of Joint Replacement Surgery and Assistant Chairman of the Department of Surgery at Shore Medical Center. “I know myself, as well as other study investigators, have identified several patients that may be candidates for this compassionate use provision. The one patient that was approved by the FDA is scheduled for the procedure at our institution in March 2025.”
But while compassionate use approval is a positive step, it is only the beginning. The orthopedic community will be watching closely as data from these cases are gathered. If the Reverse HRS can consistently demonstrate superior outcomes, this could mark the beginning of a paradigm shift in hip replacement surgery for patients with spinal fusion and spinal pelvic disorders.
HIT’s CEO, George Diamantoni, has emphasized the company’s commitment to advancing patient care, and this milestone aligns with that mission. The Reverse HRS may not only offer a viable alternative for a select group of patients but could also pave the way for broader adoption in the future. However, challenges remain: regulatory hurdles, long-term clinical data, and the eventual question of cost and accessibility.
At OrthoSpineNews, we believe that innovation must be met with rigorous scientific scrutiny and practical application. The Reverse HRS presents an intriguing solution to a well-documented problem, and its compassionate use approval is an encouraging sign. If the system lives up to its promise, it could redefine hip replacement options for a vulnerable patient population.
The orthopedic world should take note—change may be on the horizon. Anyone interested in the Reverse HRS IDE pivotal clinical trial can visit ClinicalTrials.GOV here.