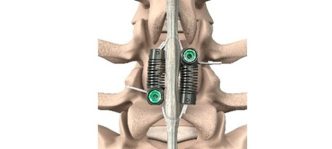
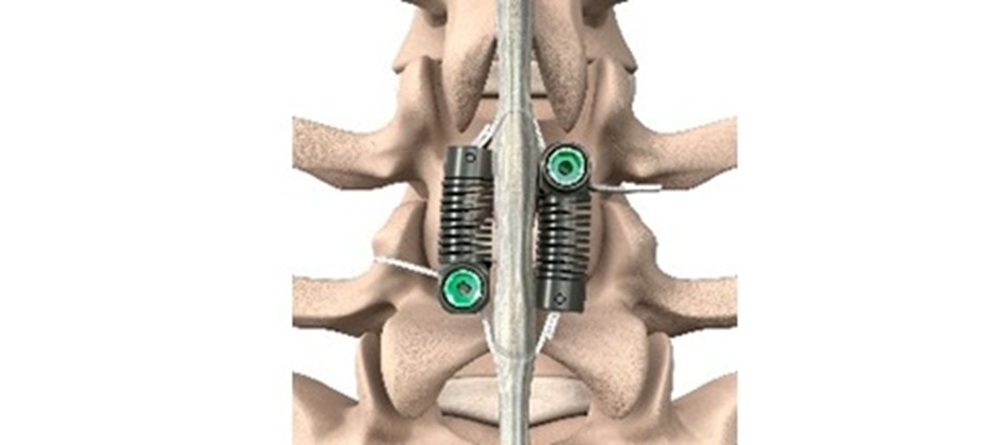
Motion-Preserving Lumbar Spine Surgery – Stabilization Without Fusion for Patients with Degenerative Spondylolisthesis
San Carlos, CA — February 18, 2026 /OrthoSpineNews/ — The U.S. Food and Drug Administration (FDA) has granted Premarket Approval (PMA) to the LimiFlex™ Dynamic Sagittal Tether, a motion-preserving system for the treatment of lumbar spinal stenosis associated with Grade I degenerative spondylolisthesis following decompression. Developed by Empirical Spine (San Carlos, CA) from foundational research at Stanford University School of Medicine, LimiFlex represents more than twenty years of refinement and clinical validation.
Degenerative spondylolisthesis affects a large and growing patient population and results in more than 250,000 surgical procedures annually in the United States, accounting for approximately half of all lumbar fusion procedures. For decades, surgeons have faced a structural treatment dilemma when treating degenerative spondylolisthesis. Decompression relieves neural compression but does not address the underlying instability, while fusion stabilizes the spine but permanently eliminates motion and increases physiologic stress on adjacent segments. As a result, many patients with this problem undergo fusion primarily to prevent instability and recurrence rather than to treat deformity.
The LimiFlex™ Dynamic Sagittal Tether stabilizes the spine following decompression without rigid fixation. By providing dynamic restraint to pathologic motion while preserving controlled movement, the procedure offers an alternative surgical strategy. Motion-preserving stabilization for these patients creates a new treatment category positioned between decompression alone and fusion. The device stabilizes pathologic motion while preserving native anatomy, maintaining a surgical magnitude similar to decompression rather than reconstruction.
In the FDA pivotal trial, LimiFlex demonstrated non-inferior 2-year clinical outcomes compared with instrumented fusion. Procedures were substantially shorter than fusion surgery and supported outpatient treatment in appropriately selected patients.
“Degenerative spondylolisthesis with stenosis causes patients severe pain and disability,” noted Co-Founder Dr. Todd Alamin, Professor of Orthopaedic Spine Surgery at Stanford University School of Medicine. “We can now relieve their symptoms with a minimally invasive procedure without the added physiologic and mechanical burden of fusion.”
“Lumbar fusion for degenerative spondylolisthesis has been a bedrock of spine surgery for decades. I have been involved in many IDE trials, and LimiFlex promises to be one of the most thoughtful advances in spine care,” said Dr. Hyun Bae, of The Spine Institute and Cedars Sinai Medical Center. “Patients always prefer motion-preserving and outpatient options, and I am excited to be able to offer this option to the community.”
“We have great outcomes with LimiFlex at our Center,” stated Dr. Rick Sasso of Indiana Spine Group, a Principal Investigator of the IDE study. “Nearly all of our patients were treated in our Ambulatory Surgery Center, and all would have otherwise had a fusion.”
Co-Founder, President and COO Louie Fielding has announced that the initial U.S. limited launch of the LimiFlex™ Dynamic Sagittal Tether will begin shortly at select medical centers that participated in our IDE trial, with a broader launch to establish additional centers of excellence later this year.
For more information, please visit https://www.limiflex.com/.
Contact
Louie Fielding
Co-Founder, President and COO
info@empiricalspine.com
650-585-6307
About Empirical Spine
Empirical Spine, Inc., based in San Carlos, California, is a medical technology company focused on developing surgical solutions that align the magnitude of treatment with the magnitude of disease. The company’s technologies are designed to address structural spine conditions using motion-preserving approaches intended to reduce the physiologic burden of traditional surgical constructs.