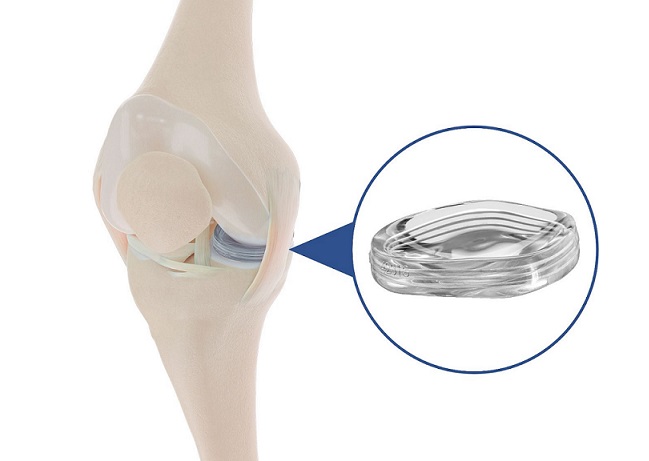
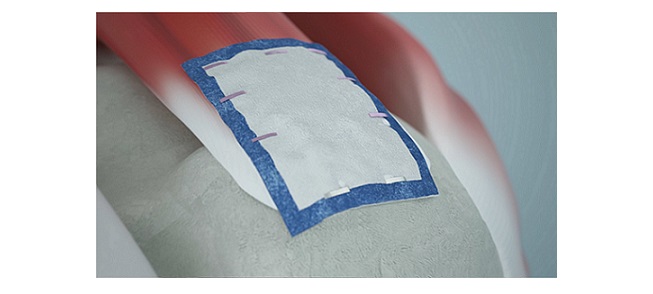
The TissueTak™* tendon anchor is the first closed-loop suture based fixation for soft tissue to soft tissue repair of certain types of injuries, designed as a less-invasive surgical system for orthopedic surgeries
HAIFA, Israel, July 2021 — Via Surgical, a leading developer of novel surgical fixation solutions, announced today it has received 510(k) clearance by the U.S. Food and Drug Administration for the TissueTak™ tendon anchor. It is the first suture-based fixation system to be used for arthroscopic rotator cuff repair and augmentation.
“In our clinical and biomechanical assessment, TissueTak showed superior strength for affixing biological patches to the tendons,” said Ofek Levin, Co-Founder and Chairman of the Board at Via Surgical. “We’re excited to get our technology into more operating rooms, so we can help improve rotator cuff surgeries for both patients and doctors.”
Rotator cuff tears are one of the most common sports injuries and often require surgery to repair the damaged torn tendon to bone. Recent innovations involving biological augmentation, where a graft is affixed to supplement the injured tendon, require a surgical staple or complex suturing techniques.
The TissueTak system utilizes a suture-based anchor to attach the patch directly to the patient’s tendon, providing a high-strength alternative to traditional surgical staples or suture techniques which are inefficient and difficult to place. Multiple fasteners are deployed using an ergonomically-shaped device that inserts each faster to affix a biological patch to the patient’s own tissue without reloading or removing the device from the joint.
Via Surgical’s fasteners are more secure than traditional staples due to a proprietary closed lock-loop suture concept, as evidenced in usability tests in cadaver tissue. The suture-like implants can be quickly and easily deployed through laparoscopic incisions. This helps make the procedure more efficient and less complex for both the doctor and operating room staff. For patients, the new system can result in a reduced amount of time in the OR, less pain, and provides a reproducible procedure to reduce retear rates following rotator cuff repair.
With the FDA clearance, Via Surgical will now be able to sell and distribute its device in the United States and in all countries that recognize FDA clearance.
“The TissueTak Tendon Anchor is very easy to use in arthroscopic surgeries,” said Brian J. Cole MD, MBA, and Professor in the Department of Orthopedics at Rush University Medical Center in Chicago. “We are always striving to improve the clinical outcomes of our patients following rotator cuff repair and technically we have not had a reliable solution to augment these repairs with a patch due to the absence of proper fixation options. The TissueTak is the first commercially available option to provide efficient mechanical fixation of a patch to the native rotator cuff tissue. Considering the fact that there are almost half a million of these procedures done each year in the U.S. alone, TissueTak holds amazing potential in the field of orthopedics.”
Gautam P. Yagnik, MD, said, “The TissueTak’s innovative technology allows for reliable and secure soft tissue to soft tissue fixation that can be performed quickly and efficiently during arthroscopic surgery. With the TissueTak we now have a safe and reliable way to augment any type of rotator cuff repair with a patch transforming the way surgeons perform arthroscopic rotator cuff surgery.”
*TissueTak™ is a trademark owned by Arthrex, Inc.





About Via Surgical

Via Surgical Ltd. provides lockable fixation solutions for soft tissue repairs. Realizing that soft tissue tends to move, shift, contract and expand, Via Surgical’s products are designed to lock into the tissue, providing a secure fixation. The company was founded in 2012 by a dedicated team with a successful track record in the hernia space. It has raised more than $12 million in funding, led by OTV VC and with an investment from Asian manufacturing giant Catcher Technology, its first in the medical device field. To date, Via Surgical has three FDA clearances, and 17 issued patents in the US and worldwide, and 17 additional provisional patents filed in the US. Via Surgical’s soft tissue fixation system was voted as one of the Top 10 Surgical Solutions by MedTech Outlook Review. The company also received the Seal of Excellence from Horizon 2020, the EU’s research and innovation funding program.