FIBERGRAFT® BG Matrix Achieved 96.3% Fusion Rate in Posterolateral Spine Fusion Clinical Study
NEW PROVIDENCE, N.J. (PRWEB) AUGUST 26, 2021
Prosidyan, Inc. (http://www.prosidyan.com), developer of proprietary fiber-based bioactive glass bone graft substitute products, announces the presentation of the “Multi-Center Clinical Evaluation of a Bioactive Fiber-Based Bone Graft Substitute for Posterolateral Fusion” clinical study poster based on its FIBERGRAFT BG Matrix product this past weekend at the American Association of Neurological Surgeons’ Annual Meeting.
The clinical study e-poster presentation details the study’s design, methods and outcomes that support the conclusion that Prosidyan’s bioactive glass fiber-based bone graft substitute achieved a successful 96.3% fusion outcome in a multi-center clinical evaluation. There were no complications or adverse events related to the device.
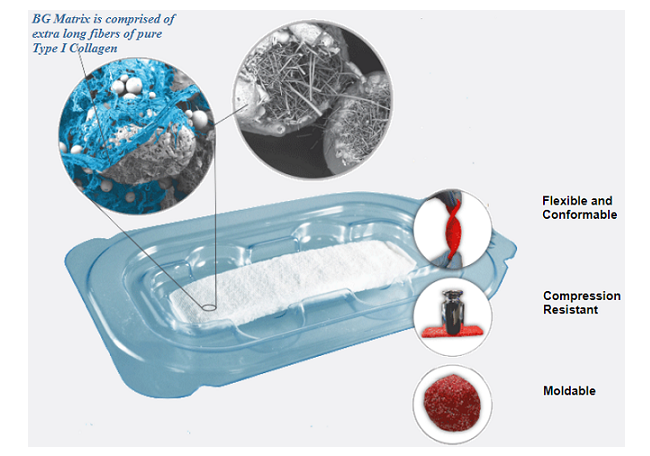
FIBERGRAFT BG Matrix is an ultraporous synthetic bone graft substitute made from proprietary bioactive glass fibers. BG Matrix is comprised of the clinically-proven FIBERGRAFT bioactive glass technology and extra-long fibers of pure type I collagen for flexibility and conformability. In additional to this clinical study, FIBERGRAFT BG Matrix has also been evaluated in a preclinical study that showed it to be equivalent to autograft in a challenging rabbit posterolateral spinal fusion model¹.
“We are proud of the results achieved in this clinical study and will continue to support the generation of clinical evidence proving the success of our bioactive glass technology in Orthopedic Trauma and Spine surgical procedures. We are motivated to continue to partner with our surgeon customers to assist them in healing patients faster and with fewer complications,” said Charanpreet Bagga, Co-Founder, President and CEO of Prosidyan, New Providence, NJ.
Within the last twelve months, there have been four FIBERGRAFT abstracts accepted for presentation at world-renowned orthopedic and spine meetings.
About Prosidyan
Prosidyan was founded in 2009 to develop a family of synthetic bioactive bone graft substitutes based on proprietary bioactive glass fibers. Prosidyan’s first product, FIBERGRAFT BG Morsels, a synthetic bone graft substitute, received FDA clearance in March 2014, and the first surgery utilizing this innovative bone graft substitute was performed in May 2014. The Company’s second product in the line, FIBERGRAFT BG Putty, received FDA clearance in March 2015, and comprises FIBERGRAFT BG Morsels in combination with Prosidyan’s proprietary bioactive carrier, OSSIGLIDE®. Prosidyan’s latest technology, FIBERGRAFT BG Matrix launched in August 2017, uses Prosidyan’s proprietary type I collagen-based bioactive carrier.
For more information about the company and its products, please visit http://www.prosidyan.com, or call 908.517.3666.
¹ Walsh WR, Oliver R, Chistou C, Wang T, Walsh ER, Lovric V, Pelletier M, Bondre S. Posterolateral fusion in a NZ white rabbit model using a novel bioglass fiber combined with autograft. Poster#390, ORS 2017 annual meeting, San Diego, 19–22 March 2017.