September 10, 2021
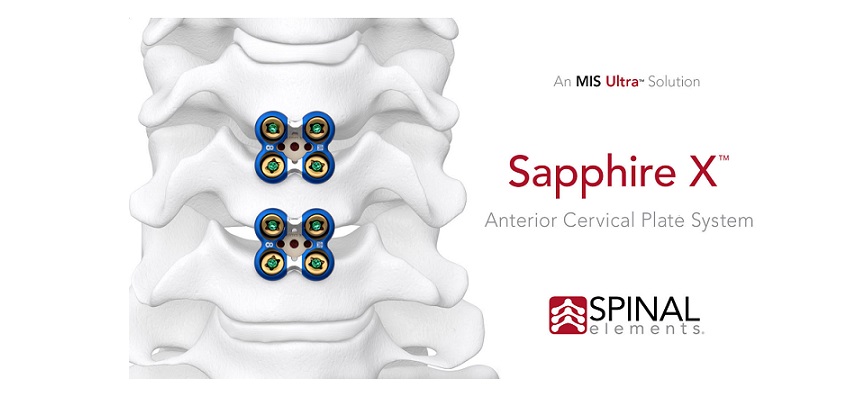
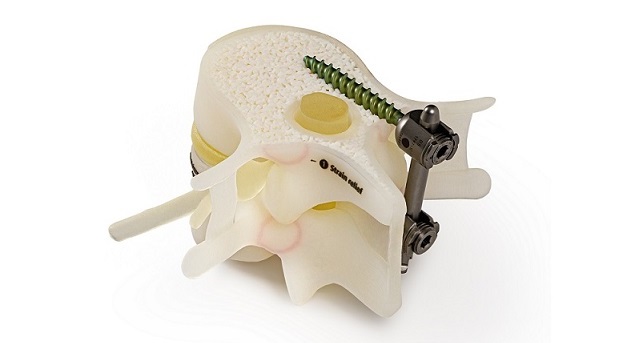
CARLSBAD, Calif.–(BUSINESS WIRE)–Spinal Elements today announced it has reached the milestone of completing 100 Sapphire X procedures after only a few weeks of commercialization. This minimally invasive surgical anterior cervical plate system features streamlined integrated instrumentation designed to increase procedural efficiency while reducing procedural steps and complexity. As one of the shortest plates on the market, Sapphire X is also designed to enhance long-term outcomes by reducing adjacent level ossification.1
Tien V. Le, M.D., FAANS, a neurosurgeon with Total Spine & Brain Institute in Tampa, FL and an expert in minimally invasive spine surgery commented after completing the 100th Sapphire X procedure, “Sapphire X is an advanced anterior cervical plating system that stands out in a saturated field. Its ease of use, versatility, and reproducibility saves time for single or multi-level cases. It also provides for an ideal option for use in adjacent segment degeneration cases.”
“We are thrilled to see Sapphire X gain exposure across the country so quickly. It’s been very satisfying to work with such an incredible group of surgeons like Dr. Le, whose collaboration has led to the rapid adoption of the Sapphire X technology,” said Jason Blain, CEO of Spinal Elements.
John DeVine, M.D., Professor and Chief of Spine Surgery at Augusta University Medical Center in Augusta, GA added, “After implanting the cage, the surgeon simply drops the plate using the integrated insertion system and secures with screws. Sapphire X makes interbody fusion and fixation the easiest part of the procedure.”
Along with the entire MIS Ultra™ suite of products, Spinal Elements will feature Sapphire X at the upcoming meeting of the North American Spine Society in Boston on September 29 through October 2, 2021.
About Spinal Elements
Spinal Elements is a Carlsbad, California based medical device company focused on the design, development, and commercialization of a comprehensive portfolio of systems, products, and technologies for spine surgery procedures. A leading designer, developer, manufacturer, and marketer of innovative medical devices used in spinal surgical procedures, Spinal Elements combines leading medical device technologies, biologics, and instrumentation to create positive surgical outcomes that exceed surgeon and patient expectations. Spinal Elements has built a reputation delivering innovative and differentiated technologies that enable fundamental shifts in solutions for spine surgery. The company markets a complete portfolio of advanced spinal implant technologies. For more information, please visit www.spinalelements.com.
Reference:
- Development of adjacent–level ossification in patients with anterior cervical plate. Park J-B, Choe Y-S, Riew KD. J Bone Joint Surg Am. 2005;87(3):558-63.
Contacts
For interviews or more information, contact:
Laura Charlton (formerly Johnson) for Spinal Elements
laurajohnsonpr@yahoo.com (760) 450-7749 cell