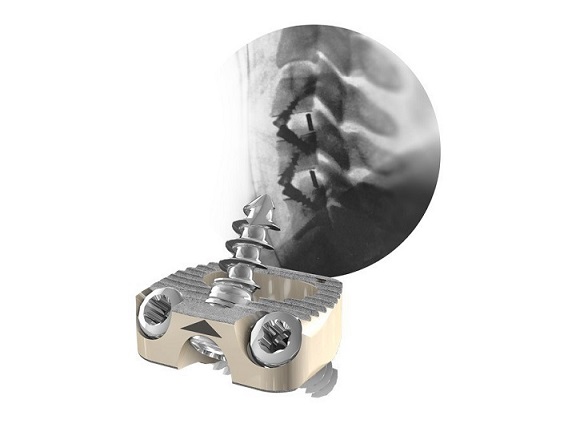
Eminent Spine’s Anterior Cervical Plate System has received 510(K) clearance from the FDA to offer a modern, low-profile option to the market. Their Cervical Plate System is accompanied by an array of instrumentation that focuses on making surgery safe and efficient.
Plano, TX, October 22, 2021 /OrthoSpineNews/ — Eminent Spine has released the clinical results of the Anterior Cervical Plating System at the 36th annual NASS conference after receiving FDA clearance in July of 2020. The alpha launch, consisting of multiple spine centers, was used in 100 cervical fusions. 100 anterior cervical plates and 630 screws were applied, with no cervical plates or screws experiencing breakage and no screw backout.
The cervical plate was designed thin and low-profile, with self-tapping screws that have a pointed tip that make insertion easy. There are two diameters built into the screwdriver, which allows for less instrumentation usage as well as increased efficiency and safety in the OR. Surgeons are enthusiastic about the straightforward nature of the system and the reliability of the locking mechanism.
The cervical plate is accompanied by Eminent Spine’s cervical IBFD cages, which are available in PEEK, Titanium, and 3D Printed Titanium* (*Pending 510K approval).
Eminent Spine is a medical device manufacturing company that focuses on anticipating and adjusting to the changing needs and desires of the industry in order to bring its customers the best products on the market.
If you would like more information about this topic, please call DAGEN HYBNER at 972-499-3593, or email INFO@EMINENTSPINE.COM.