Implant Also Receives a 2021 Popular Science “Best of What’s New” Award in Health
December 1, 2021
WESTBOROUGH, Mass.–(BUSINESS WIRE)–Miach Orthopaedics, Inc., a privately held company dedicated to developing bio-engineered surgical implants for connective tissue restoration, today announced the BEAR® Implant is now commercially available in select U.S. cities for the treatment of anterior cruciate ligament (ACL) tears, one of the most common knee injuries in the U.S. The BEAR Implant was also awarded a Popular Science Best of What’s New Award for representing a significant step forward in health.
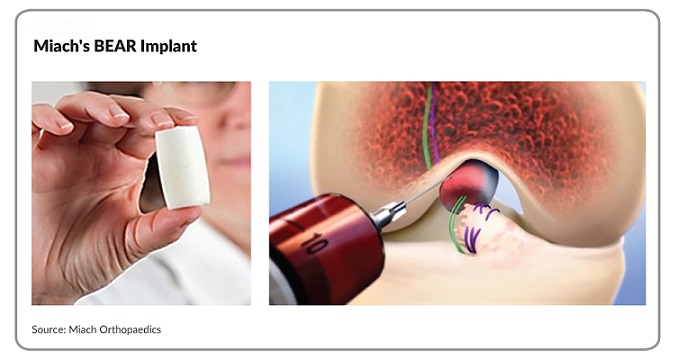
The BEAR Implant is the first medical technology to clinically demonstrate that it enables healing of a patient’s torn ACL. It is a paradigm shift from the current standard of care – reconstruction that replaces the ACL with a graft.
“Patients will be very interested in having another option for treating ACL injuries. This is the first FDA-approved implant to clinically demonstrate that it enables healing of the patient’s native torn ACL while maintaining the natural knee anatomy,” said Dr. Jonathan L. Glashow, clinical assistant professor, NYU Grossman School of Medicine, and chief medical advisor for the NJ Devils and Philadelphia 76ers. “I’m encouraged by the clinical studies that have demonstrated appropriate patients experienced faster recovery of muscle strength and higher satisfaction with regard to readiness to return to sport than others with traditional ACL reconstruction.”
The proprietary, bio-engineered BEAR Implant has several benefits over ACL reconstruction: It restores ACL quality and size similar to the non-injured ACL, results in faster recovery of muscle strength and has better patient satisfaction with being ready to return to sports. The BEAR Implant also does not require a graft, eliminating the need for a second wound site to heal and worries about donor graft quality or risk of disease.
“ACL tears are a common sports injury, no matter what you play or at what level, and they can be devastating for our players in the NFL,” said Dr. Thom Mayer, who serves as medical director for the NFL Players Association. “As a new treatment option with exciting benefits over traditional ACL reconstruction, I’m hopeful that the BEAR Implant can help people return to the sports and activities they love.”
Every year, approximately 400,000 ACL injuries occur in the U.S. A torn ACL does not heal on its own, resulting in ACL reconstruction being one of the most common orthopedic procedures in the U.S.
“We’ve been overwhelmed by the interest from patients who’ve torn their ACLs and are actively seeking an alternative to the current standard of care,” said Martha Shadan, president and CEO, Miach Orthopaedics. “We’re honored the BEAR Implant was selected by Popular Science as one of the best new health products of the year, and we are excited to make this innovative treatment available for patients.”
To find a surgeon who offers the BEAR Implant, visit miachortho.com.
About The BEAR® Implant
The Bridge-Enhanced® ACL Restoration (BEAR®) Implant is a proprietary bio-engineered implant used to facilitate healing of the torn ACL. Unlike reconstruction, which is the current standard of care, the BEAR Implant does not require a second surgical wound site to remove a healthy tendon from another part of the leg or the use of a donor tendon. The BEAR Implant acts as a bridge between the two ends of the torn ACL. The surgeon injects a small amount of the patient’s own blood into the implant and inserts it between the torn ends of the ACL in a minimally invasive procedure. The combination of the BEAR Implant and the patient’s blood enables the body to heal the torn ends of the ACL back together while maintaining the ACL’s original attachments to the femur and tibia. The BEAR Implant is absorbed by the body as the ACL heals.
The BEAR Implant was granted De Novo Approval from the U.S. Food and Drug Administration in December 2020. It is indicated for skeletally mature patients at least 14 years of age with a complete rupture of the ACL, as confirmed by MRI. Patients must have an ACL stump attached to the tibia to facilitate the restoration. The BEAR device must be implanted within 50 days of injury. Visit miachortho.com for complete product information, including Instructions for Use.
About Popular Science Best of What’s New
Every year since 1988, the editors of Popular Science have reviewed thousands of products in search of the top 100 innovations—breakthrough products and technologies that represent significant advancements in their categories. Best of What’s New Awards are presented to products and technologies in 10 categories, including health.
About Miach Orthopaedics, Inc.
Miach Orthopaedics, Inc. is a privately held company located in Westborough, Massachusetts, dedicated to developing bio-engineered surgical implants for connective tissue restoration. The company’s initial focus is the Bridge-Enhanced® ACL Restoration (BEAR®) Implant, which represents a paradigm shift in the treatment of ACL tears from reconstruction to restoration. The BEAR technology was pioneered by Martha Murray, M.D., founder of Miach Orthopaedics, at the Boston Children’s Hospital Department of Orthopaedic Surgery, with initial research funding provided by the NFL Players Association, Boston Children’s Hospital and the National Institutes of Health. For more information on Miach Orthopaedics and its products, visit www.miachortho.com and follow the company on Facebook, Twitter and LinkedIn.
Contacts
Joni Ramirez
Merryman Communications
joni@merrymancommunications.com
323-532-0746