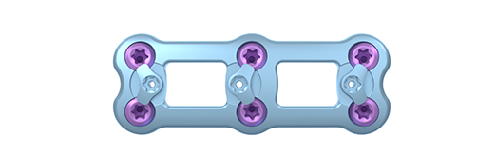
SHELTON, Conn., Dec. 02, 2021 (GLOBE NEWSWIRE) — Spine Wave is pleased to announce the immediate launch of both the Defender® Anterior Cervical Plate, and the Stronghold® C 3D Titanium Interbody Device featuring TiCell® 3D advanced surface technology. The Defender® Anterior Cervical Plate is a titanium plate and screw system that provides fixation for anterior cervical fusion procedures. The Stronghold® C 3D Titanium Interbody Device is a cervical interbody fusion implant manufactured using 3D printing methods to produce a unique surface designed to optimize the implant’s interface with bone. These exciting new products substantially strengthen Spine Wave’s position in the important anterior cervical fusion market segment.
The Defender® Anterior Cervical Plate is a “workhorse” titanium anterior cervical fixation system for anterior cervical fusion. It provides secure fixation by offering fixed and variable angle screws in a dual- lead thread design in multiple diameters with a wide range of lengths, and in both self-drilling and self-tapping designs. Simple locking is provided with a dual-cam locking mechanism that secures both screws at each level in one step. The Defender® Anterior Cervical Plate is also designed with large graft windows to provide direct visualization during the procedure and to facilitate radiographic visualization after the procedure.
The Stronghold® 3D Titanium Interbody Device is offered in a wide range of sizes to comprehensively address the requirements of cervical interbody fusion procedures. The TiCell® 3D advanced surface technology featured on all Stronghold® 3D Titanium Interbody Device implants is characterized by a dual layer of optimally sized interconnected pores. This interconnected porous structure is constructed in a lattice intended to mimic the organic structure of cancellous bone and provide a rough surface to strengthen the interface between implant and bone. All Stronghold® 3D Titanium Interbody Device implants, which are also offered in TLIF and PLIF designs in addition to Cervical, also feature an open architecture to contain large amounts of bone graft and facilitate fusion.
“Defender® Anterior Cervical Plate together with Stronghold® C 3D Titanium Interbody Device is a great alternative for my anterior cervical fusions,” said Julio Petilon, M.D. “The Defender® Anterior Cervical Plate comprehensively meets my clinical needs and I use it with confidence because the dual-cam locking mechanism is secure and easy to use. Importantly, the same screwdriver is used to insert and lock the bone screws and that can save time in my procedures.” He continued, “the TiCell® 3D advanced surface technology featured on Stronghold® C 3D Titanium Interbody Device implants is designed to provide an excellent setting to achieve fusion.” Dr. Petilon is a board-certified orthopedic spine surgeon practicing with Atlanta Bone and Joint Specialists in Atlanta, Georgia.
“Spine Wave is executing its plan to become a full line spine company and a preferred choice for spine surgeons and spine distributors,” said Laine Mashburn, Spine Wave’s Executive Vice President for Global Marketing and Business Development. The Defender® Anterior Cervical Plate and the Stronghold® C 3D Titanium Interbody Device each address important market segments with advanced technology and appealing features. Both products are very complementary to our Proficient® Posterior Cervical Spine System which has been very well received in the market. Our cervical fusion portfolio has never been so well positioned and capable of driving new growth for Spine Wave.”
About Spine Wave
Spine Wave is a leader in minimally invasive spine surgery and expandable interbody devices. The company is committed to offering a broad portfolio of differentiated product and procedure solutions for spine surgeons and their patients. In addition to the Defender® Anterior Cervical Plate and the Stronghold® C 3D Titanium Interbody Device, Spine Wave offers a broad portfolio of advanced spine implant and biologic products. The company is expanding rapidly and continues to recruit sales managers and independent distributors to fuel growth. For more information on Spine Wave and its products please visit www.spinewave.com.
Contact
Laine Mashburn, Executive Vice President, Global Marketing and Business Development
lmashburn@spinewave.com
(203) 712-1863
Terry Brennan, Chief Financial Officer
tbrennan@spinewave.com
(203) 712-1810