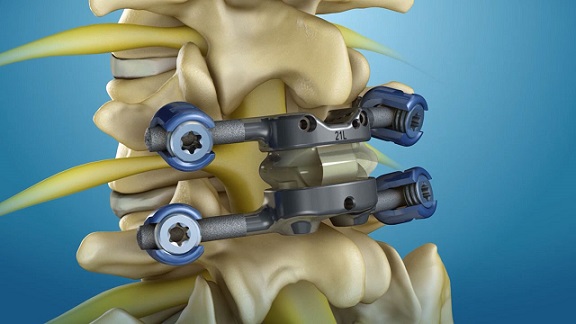
NEW YORK, June 1, 2022 /PRNewswire/ — A multicenter, prospective, randomized controlled trial is designed to assess the effectiveness of three different disc replacement devices used for two-level symptomatic cervical disc disease. The study is sponsored by Centinel Spine, LLC, and Hospital for Special Surgery (HSS) in New York City, is one of the centers participating in the research.
Cervical disc disease, or degenerative disc disease, is the progressive deterioration of spinal discs and arthritic changes in the facet joints. During this procedure, surgeons will test the safety and efficacy of removing discs at two levels and implanting an artificial disc device at each level of the cervical spine between the vertebrae where the disc was removed. The artificial disc will serve to stabilize the spine while preserving greater motion than a conventional fusion surgery.
Previous patients who underwent artificial disc replacement reported greater improvements in their pain relief, mobility, and activities of daily living than those who underwent spinal fusion surgery, the standard treatment. It is less invasive but as effective as spinal fusion surgery, however the procedure using this particular device has not been validated through a clinical trial to date.
“The concern with doing a fusion type operation is that over the long term the other discs have to accommodate for the motion lost at the fused level,” said Darren Lebl, MD, a minimally invasive spine surgeon who is leading the research at HSS. Over time, the increased mechanical demand on the surrounding vertebrae can make the discs in the region more vulnerable to additional degeneration.
“We do know very clearly that fusion is limiting motion,” Dr. Lebl added. “The concept of the two-level disc replacement is to preserve motion with two moving mechanical devices.”
To be eligible for participation, patients must be between the ages of 18 and 69 years and have received a diagnosis of radiculopathy (impingement of the nerves in the spinal column) or myelopathy with radiculopathy, a more severe form of damage to the spinal cord involving vertebrae in the neck, defined at cervical discs C3 through C7. Patients must also be experiencing symptoms associated with disc problems, such as neck or arm pain, decreased muscle strength and abnormal reflexes that has not responded well to conservative treatments such as rest, heat, physical therapy and pain relievers.
The trial will include at least 390 patients once enrollment is complete. Patients will receive regular evaluations for two years after surgery and may be examined in periodical follow-up visits for a total of seven years.
For more information about the trial at HSS, contact Philip Paschal at paschalp@hss.edu or 917-260-4285.mailto:
About HSS
HSS is the world’s leading academic medical center focused on musculoskeletal health. At its core is Hospital for Special Surgery, nationally ranked No. 1 in orthopedics (for the 12th consecutive year), No. 4 in rheumatology by U.S. News & World Report (2021-2022), and the best pediatric orthopedic hospital in NY, NJ and CT by U.S. News & World Report “Best Children’s Hospitals” list (2021-2022). In a survey of medical professionals in more than 20 countries by Newsweek, HSS is ranked world #1 in orthopedics for a second consecutive year (2022). Founded in 1863, the Hospital has the lowest complication and readmission rates in the nation for orthopedics, and among the lowest infection rates. HSS was the first in New York State to receive Magnet Recognition for Excellence in Nursing Service from the American Nurses Credentialing Center five consecutive times. An affiliate of Weill Cornell Medical College, HSS has a main campus in New York City and facilities in New Jersey, Connecticut and in the Long Island and Westchester County regions of New York State, as well as in Florida. In addition to patient care, HSS leads the field in research, innovation and education. The HSS Research Institute comprises 20 laboratories and 300 staff members focused on leading the advancement of musculoskeletal health through prevention of degeneration, tissue repair and tissue regeneration. The HSS Innovation Institute works to realize the potential of new drugs, therapeutics and devices. The HSS Education Institute is a trusted leader in advancing musculoskeletal knowledge and research for physicians, nurses, allied health professionals, academic trainees, and consumers in more than 145 countries. The institution is collaborating with medical centers and other organizations to advance the quality and value of musculoskeletal care and to make world-class HSS care more widely accessible nationally and internationally. www.hss.edu.
SOURCE Hospital for Special Surgery