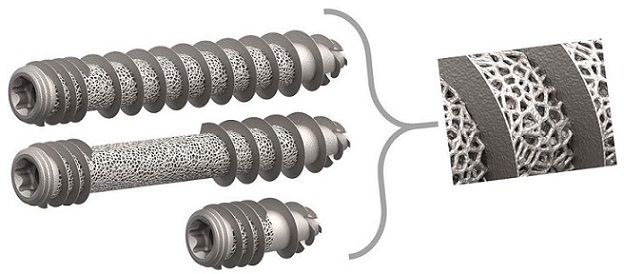
The T-FIX 3DSI Joint Fusion System is the first of many systems from Cutting Edge Spine to implement proprietary trabecular fixation platform.
MINERAL SPRINGS, NC., July 14, 2022 /OrthoSpineNews/ — Cutting Edge Spine (CES), a leader in the organic development and commercialization of bioactive implant technologies for the spine, today announced the FDA 510(k) clearance of its proprietary SI Joint Fusion System, T-FIX 3DSI Joint Fusion System.
The 3D-printed T-FIX 3DSI Joint Fusion System is part of the novel EVOL-SI Fusion System portfolio, which is designed to treat dysfunctions of the sacroiliac joint. The T-FIX 3DSI Joint Fusion System is FDA 510(k) cleared for multiple approaches: Lateral, S2Al (S2 Alar Iliac), Open Posterior Bridging, and Transgluteal Lateral. The system’s trabecular technology promotes optimal osseointegration, positively impacting fusion and fixation.
“FDA 510(k) clearance of this trabecular technology is the culmination of five years of internal development in the pursuit of higher quality patient care through solid science,” said CES President, CEO & Founder, Randy Roof. “Raising the bar in patient care continues to be our core objective, and the addition of the T-FIX 3DSI Joint Fusion System to our EVOL-SI Fusion System delivers an incredibly novel and broad system for treating dysfunctions of the sacroiliac joint.”
Established in 2009, Cutting Edge Spine is a proven leader in the organic development and commercialization of novel bioactive spinal implant systems focused on providing an optimal fiscal value proposition.
“T-FIX” and “EVOL” are registered trademarks of Cutting Edge Spine, LLC.
Cutting Edge Spine, LLC.
6012 Waxhaw Highway
Mineral Springs, NC 28108
R.Roof@cuttingedgespine.com
(704) 243-0892