Launch represents the company’s eighth commercial release within its line of Tranquil™ interbody fusion implants
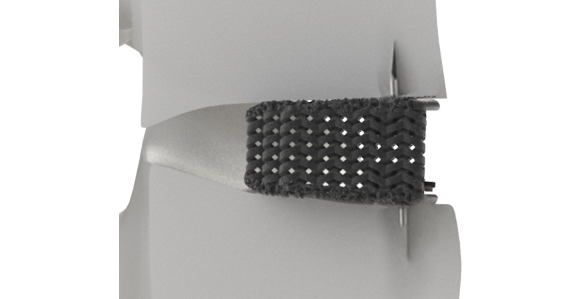
July 20, 2022—SALT LAKE CITY–(BUSINESS WIRE)–Nexus Spine, a developer of biomechanically-advanced solutions for spinal pathologies, today announced the beta launch of its Stable-C™ cervical interbody fusion implants featuring integrated anchoring blades. The limited release adds to the company’s growing line of Tranquil™ interbody offerings, which currently includes ALIF, PLIF, TLIF, Steerable TLIF, DLIF, and standard cervical configurations. Tranquil™ is made of titanium that is engineered using proprietary compliant mechanism principles to match the stiffness of spinal trabecular bone to encourage rapid stability while minimizing the potential for subsidence.
The first beta surgery was performed by Kirk Clifford, MD at Community Hospital in Grand Junction, Colorado on July 12, 2022. “Stable-C™ is an incredibly elegant system,” commented Dr. Clifford. “The integrated anchoring blades were easily deployed in one step by simply rotating an instrument that attaches to the inserter. I found it to be a big improvement over standard systems that use separate fixating components that add time and fiddle factor to my surgeries. I have been using various configurations of the Tranquil™ product line for quite some time and have experienced excellent results. My patients report early postoperative clinical improvement with radiographs that demonstrate rapid Tranquil™ integration and dynamic stability. I am excited to add Stable-C™ into my practice.”

Stable-C™ was designed by Jeffrey Hoskins, MD of the Orthopaedic Institute of Dayton, Ohio with the aim of combining intraoperative ease of use with robust fixation. “I believe we accomplished our goals with the recent tweaks we have made to the system,” commented Dr. Hoskins. “The anchoring and deployment design features are a perfect complement to the inherent advantages of the Tranquil™ matrix. I expect my Stable-C™ patients to do extremely well.”
About Compliant Mechanisms
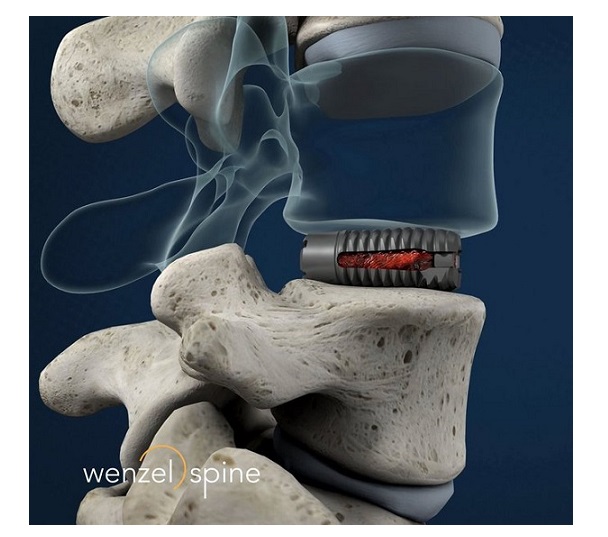
Compliant mechanisms mimic nature by providing motion and force transmission through flexibility rather than from traditional rigid-body joints. This breakthrough enables bio-friendly synthetic materials such as titanium to perform more like surgically treated tissues at the macro and microscopic levels. This approach utilizes modern mathematical modeling, 3D finite elemental analysis, and 3D printing to drastically improve the way that spinal implants perform. Nexus compliant mechanism-based devices have been implanted in patients since 2015 with the goal of achieving faster healing with less pain.
About Tranquil™ Interbody Fusion Technology
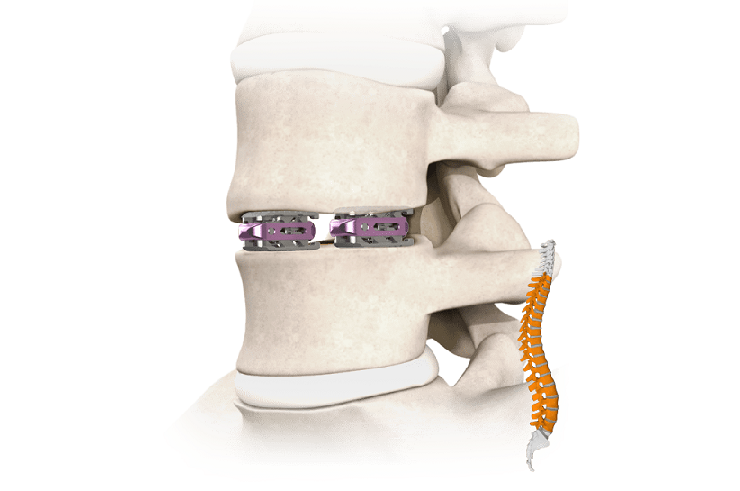
Tranquil™ is a flexible interbody fusion device made of titanium that is shaped and engineered using compliant mechanism principals to behave like spinal trabecular bone by mimicking its stiffness, resulting in a device that is 1/10th the stiffness of traditional competitive interbody fusion implants. Tranquil™ is available in cervical, ALIF, PLIF, TLIF, steerable TLIF, and DLIF configurations.
About Nexus Spine
Nexus Spine develops industry-leading spinal implants by leveraging our novel compliant mechanism engineering expertise. Our innovative and evidence-based approach continues to advance the standard of surgical technologies through a rigorous focus on improving clinical outcomes while being easier to implant. Our streamlined spinal fusion systems minimize implant bulk, surgery time, and hospital handling, which make them especially well suited for the ambulatory surgery center setting. We are wholly owned by Crocker Ventures, an independent, privately-held life science, healthcare and technology investment firm. For more information on Nexus Spine, Tranquil™ and PressON™, please visit: www.nexusspine.com.
Contacts
Andrew Shepherd
andrew.shepherd@nexusspine.com