July 20, 2022
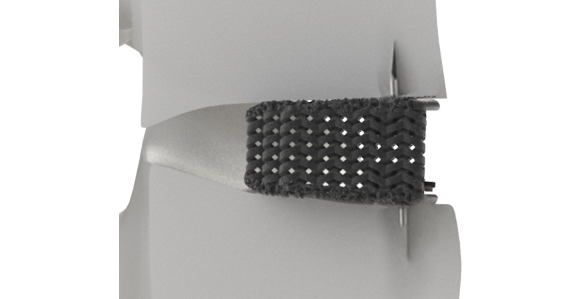
CARLSBAD, Calif.–(BUSINESS WIRE)–Spinal Elements, a spine technology company, today announced Dr. Pierce Nunley, from Specialists Hospital of Shreveport, completed the first two commercial cases with the Karma® MIS system. The Karma MIS system is designed to access and deliver the Karma implant through a 25mm paramedian incision using a single portal, streamlining the procedure, and minimizing the steps.
Karma is a metal-free cortico-pedicular posterior fixation system. Karma minimizes tissue trauma without the use of screws and rods while providing similar fixation to a pedicle screw system. The Karma MIS system provides a simple reproducible procedure using a single set of instruments and a universal PEEK strap implant. Karma’s extremely low-profile implant compares favorably versus larger metal posterior fixation devices. The system is part of Spinal Elements’ MIS Ultra™ suite of products that are intended to address the unintended consequences of spine surgery.
Jason Blain, CEO of Spinal Elements, stated, “We are excited to launch our new and innovative Karma MIS instruments. Traditional pedicle screws are state of the art for instability and deformity yet perhaps imperfect for degenerative disease indications in the lumbar spine when combined with a robust anterior column reconstruction. We believe surgeons and patients alike will notice the value and effectiveness of Karma MIS system as an alternative to metal posterior spinal fixation. As more procedures are done in surgery centers and on an outpatient basis, we think Karma’s unique characteristics and minimal instrumentation required for surgical implantation will make it an attractive solution.”
Dr. Pierce Nunley M.D., Orthopedic Surgeon and Director of the Spine Institute in Shreveport, LA. added, “Karma technology is truly a disruptive procedure in supplemental fixation. It has been an exciting journey to work with a great company and engineers to simplify the technique and allow for the device to be safely and reproducibly deployed using minimally invasive surgery techniques. Congratulations to the team for all of their hard work to reach this milestone.”
The Karma fixation system has been organically developed by the Spinal Elements team guided by minimally invasive inputs from nationally recognized spine surgeons.
About Spinal Elements
Spinal Elements is a technology-driven company headquartered in Carlsbad, California. A leading designer, developer, manufacturer, and marketer of innovative medical devices used in spinal surgical procedures, Spinal Elements combines leading medical device technologies, biologics, and instrumentation to create positive surgical outcomes that exceed surgeon and patient expectations. Spinal Elements has built a reputation delivering innovative and differentiated technologies that enable fundamental shifts in solutions for spine surgery. The company markets a complete portfolio of advanced spinal implant technologies. For more information, please visit www.spinalelements.com.
Contacts
For interviews or more information, contact:
Laura Charlton (formerly Johnson) for Spinal Elements
laurajohnsonpr@yahoo.com (760) 450-7749 cell