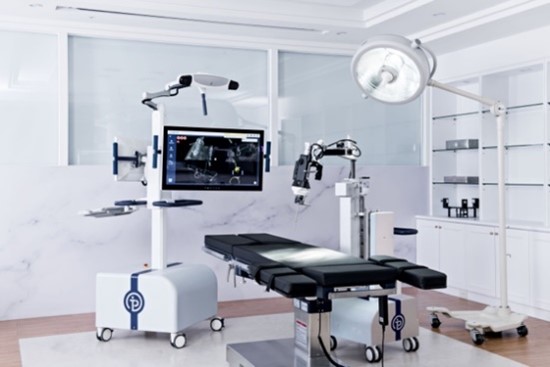
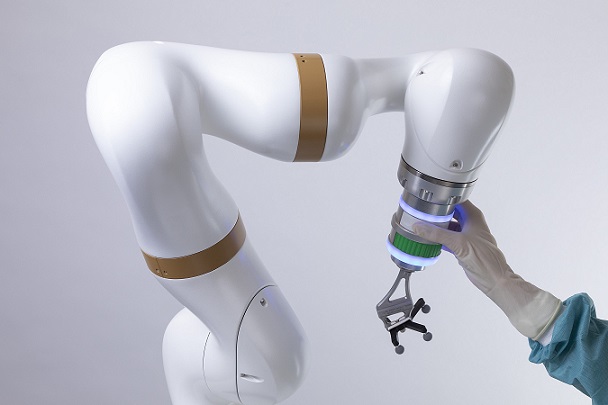
August 23, 2022 – Point Robotics MedTech Inc., /OrthoSpineNews/ – (Point Robotics), a rising leader in the field of minimal invasive orthopedic surgical robots, today announced that it has received 510(k) clearance from the Food and Drug Administration (FDA) in the United States for its minimally invasive surgical robot POINT™ Kinguide Robotic-Assisted Surgical System. Point Robotics’ approval marks both Taiwan’s very first FDA-cleared surgical robot and the first ever hand-held robot framework equipped with parallel manipulator for orthopedic application in the world.
“Precise positioning is the crucial factor for a successful spinal surgery,” said SC Juang, CEO of Point Robotics. “Wider application of orthopedic surgical robots in the spine, joints, and trauma surgeries will be the development trend in the coming decade. Following the clearance, Point Robotics is preparing for premarket submissions in Europe and China to jump start global deployments and to access international markets.”

Embedded with the high precision and unique hand-held robot, the “Kinguide Robotic-Assisted Surgical System” assists the surgeon to drill bone and implant screws more efficiently and effectively than ever. With a fully-integrated surgical system that includes both navigation system and handheld drilling system, the “Kinguide Robotic-Assisted Surgical System” streamlines procedural tasks and significantly reduce surgeon’s burdens during implant surgery. Point Robotics next plans to expand the indication of the Kinguide Robotic System to other spinal surgery procedures, including herniated disc decompression, so patients can receive safer and more effective treatments.
Wen-Cheng Luo, Chief of Neurosurgery Department at Taipei Medical University Hospital, a leading medical center in Taiwan, who has performed over 8,000 spinal surgeries, commented: “There is still a huge unmet clinical need for spinal surgical robots. It will be a milestone in minimal invasive spinal surgery method once a revolutionary new product having high degree of freedom and versatility can not only significantly reduce surgeons’ burden of clinical procedures such as implant positioning, but also meet the growing demand for decompression surgery.”
“We are extremely excited for Point Robotics to receive 510(k) clearance for their minimally invasive spinal surgery robot,” says Michael Huang, Point Robotics’ board member and managing partner of Taiwania Capital. “Receiving the 510(k) clearance marks the beginning of the new chapter for Point. With the versatility of Point’s technology platform, we believe Point Robotics has the potential to become the global leader in minimally invasive spinal surgery.”
“Point Robotics is revolutionizing surgery as we know it, by combining the medical know-how garnered from years of experience and cutting-edge technology. We are pleased to work with the company to bring groundbreaking advancements to the US with swift FDA clearance — something that has indicated the products thoroughness in design and high efficacy.” Says Jackie Yang, the Co-Founder and Managing Director of Translink Capital, another board member of Point Robotics.
To tap into the product’s market potential and resources, Point Robotics will be conducting a month-long product trials for clinicians on the US West Coast starting August 22nd and will debut the “Kinguide Robotic-Assisted Surgical System” at the following medical conferences: SMISS (9/29-10/1, Las Vegas, at booths 107 & 109), NASS (10/12-10/14, Chicago, at booth 5011), and EUROSPINE (10/19-10/21, Milan, at booth 102). For more information, visit https://www.pointroboticsinc.com
About Point Robotics MedTech Inc.
Point Robotics MedTech, a pioneer in minimally invasive surgical technologies, was founded in Taiwan since 2016. The Company has two major product lines: its “Kinguide Robotic-Assisted Surgical System” and “Kinguide Agile Hybrid Navigation System. In May 2020, the company completed its US$18 million series-A fundraising from government funds, Taiwania Capital, and Translink Capital. Point Robotics MedTech will continue to focus on the development and regulatory approvals of next-generation products to maintain its leading position in the high-end surgical robotics market.
Victoria Huang
Point Robotics MedTech Inc.
ir@pointroboticsinc.com