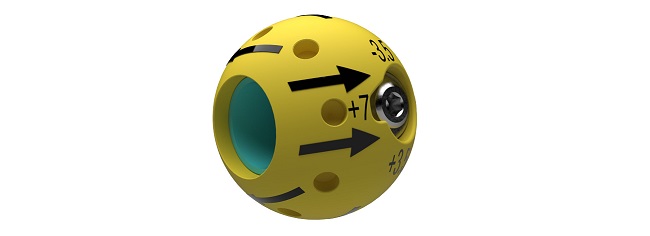
November 1, 2022
BELLEVUE, Wash.–(BUSINESS WIRE)–CurvaFix, Inc., a developer of medical devices to repair fractures in curved bones, today announced it has received 510(k) clearance (K222505) from the U.S. Food & Drug Administration (FDA) for its smaller-diameter CurvaFix® IM Implant indicated for fixation of fractures of the pelvis. The new 7.5mm device is designed to simplify surgery and to provide strong, stable, curved fixation in smaller patients.
“With this latest FDA clearance, we are able to offer surgeons the CurvaFix Implant in two diameters, 7.5mm and 9.5mm, and in lengths ranging from 90mm to 180mm,” said Steve Dimmer, chief executive officer. “Surgeon feedback from our first year of commercialization has told us that a device for pelvic fractures in narrow, curved pelvic corridors was also needed. Both smaller high-impact trauma patients and petite fragility fracture patients should benefit from this new, sleeker, curved implant. This is a key milestone in our mission to simplify pelvic fracture fixation surgery for orthopedic trauma surgeons and to help patients mobilize soon after surgery.”
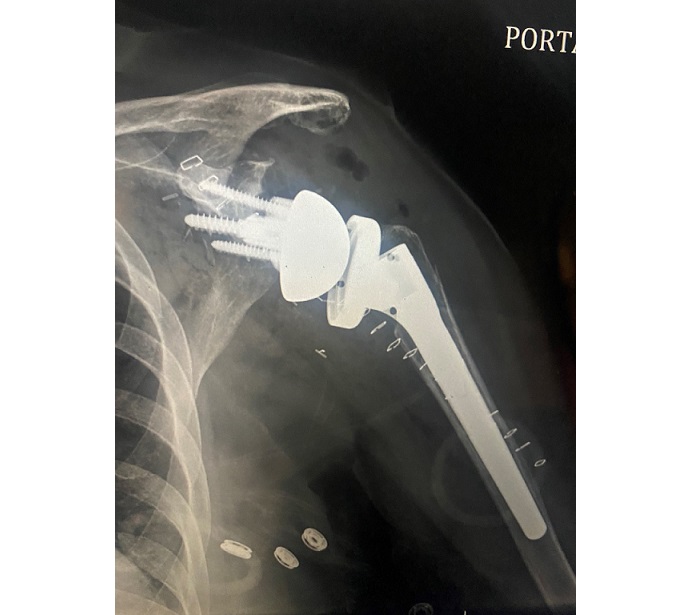
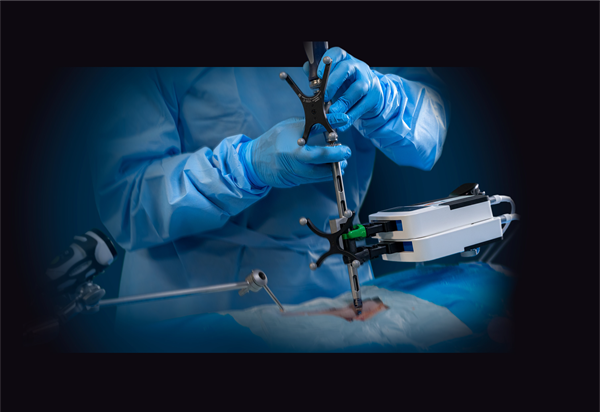
Clinical case reports have indicated that the CurvaFix IM Implant achieves strong, stable fixation that follows and fills the natural curvature of each patient’s anatomy, which may immediately reduce pain, allow for earlier mobility, and improve patient recovery. The CurvaFix IM system simplifies pelvic fracture fixation surgery with a fast, easy, repeatable, minimally invasive procedure. The system has been shown to address many pelvic fracture fixation challenges, including dysmorphic sacra, curved superior rami, and to enable surgeons to address challenges of fragility fractures of the pelvis (FFP) in geriatric patients by providing strong fixation in weak bone with a minimally invasive procedure.
There are over 186,000 hospitalizations for pelvic fractures in the U.S. every year. Due to an aging population, the incidence is growing at 9% per year. Of the hospitalized, 108,000 are due to FFP injuries in geriatric patients, 80% of whom are female.
FFPs can dramatically change the quality of life for geriatric patients and their families due to a loss of patient autonomy, significant disability, and even death. Despite recommendations that surgical treatment should be considered for most pelvic fragility fractures, only 10% receive surgery today. For non-operative patients, conservative treatment generally consists of bed confinement, pain control, and mobility assistance while tolerating weight-bearing. Often, conservative treatment leads to lengthy hospitalizations, high nursing home admittance, and a high one-year mortality rate. In contrast, decades of innovation in hip fracture repair have enabled strong, stable surgical fracture fixation to become the standard of care. Ninety-five percent of hip fracture patients receive surgery today, which can greatly reduce pain and often allows geriatric hip fracture patients to mobilize soon after surgery.1,2
About CurvaFix, Inc.
CurvaFix, Inc. is a privately held medical device company headquartered in Bellevue, Wash. CurvaFix is developing implantable products to improve fracture repair in curved bones. The company is focusing on Fragility Fractures of the Pelvis and high-impact pelvic fractures. The CurvaFix IM Implant has received 510(k) clearance from the U.S. Food & Drug Administration (FDA).
CurvaFix is a trademark of CurvaFix, Inc., registered in the U.S.
__________________________________
1 Orthopedic News Network, Vol 32, No 2, May 2022
2 Rommens P. A., et. al., Classification of fragility fractures of the pelvis: recommendations for surgical treatment, Injury, June, 2013
Contacts
Amy Cook
Acook@curvafix.com