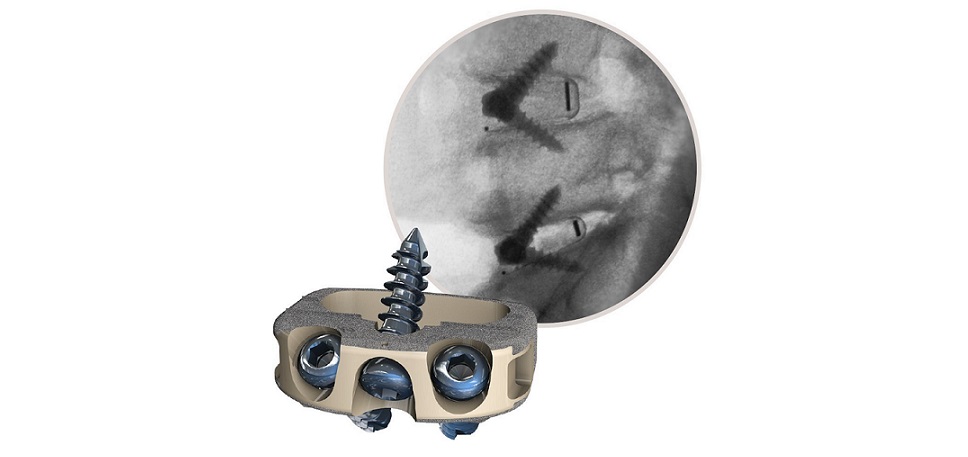
Eminent Spine’s ALIF Stand-Alone System includes PEEK and 3D Printed Titanium footprints. The implants are offered as non-sterile.
Plano, TX, November 28, 2022 /OrthoSpineNews/ — Eminent Spine received 510(K) approval of their ALIF Stand-Alone System as of October 17, 2022. The ALIF implant was designed with the following: a tapered nose which allows for ease of insertion, lordosis for ease of insertion, self-distraction, and aggressive teeth for implant fixation. Micro-teeth on the top faces of the implant prevent micro-migration and micro-motion. The locking tab shows visible security of self-tapping, self-drilling screws. There is one universal driver for screws and locking tab for ease in the OR. Surgeon’s feedback has been positive regarding the simplicity of the instrumentation and numerous footprints offered.
Eminent Spine’s ALIF Stand-Alone System includes non-sterile implants, offered in 30° hyperlordotic angles and 5 implants profiles. The ALIF Stand-Alone System is accompanied by Eminent Spine’s unique surface technology, the cortical-cancellous triple lead MIS pedicle screw system.
Contact
Eminent Spine, LLC
Dagen Hybner
972-499-3593
www.eminentspine.com