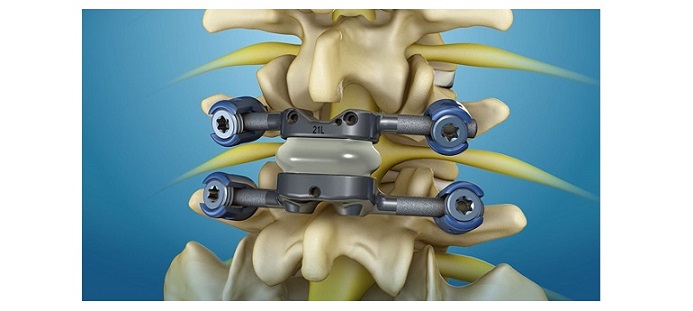
DALLAS, TX, February 20, 2023 /OrthoSpineNews/ – Osseus, an innovative spinal solutions company, announces that the Pisces-SA has been granted the first ever FDA standalone indication for an integrated ALIF interbody utilizing alternative fixation (anchors) without the need for supplemental fixation.
The primary goals of integrated fixation in ALIF interbodies are to prevent interbody expulsion and stabilize the spinal segment. Traditionally, screws have been used as the integrated fixation method for standalone devices. However, inserting these screws can be challenging due to extreme insertion angles and anatomical impediments. There are also alternative fixation methods such as anchors, blades, etc. that can be implanted through specialized inserters which allow for a minimally invasive, direct anterior approach with no need for additional instrumentation to overcome angulation challenges.
Dr. Michael Hisey, an orthopedic surgeon with Texas Back Institute in Plano, TX, describes the system’s versatility, “I’ve used screws […], and I’ve used the blade-type constructs, but I’ve never used a device where you could make that choice intraoperatively. I really like that ability to make my decision at the time.”
Although these alternative fixation methods are quicker and easier to insert, until now with the Pisces-SA, no integrated ALIF interbody utilizing alternative fixation has been approved by the FDA as a standalone device. This means that supplemental fixation (e.g., facet screws or posterior fixation) must be implanted in a secondary procedure to work in conjunction with the integrated ALIF device to adequately stabilize the spinal segment. Osseus performed and presented the FDA with extensive biomechanical testing to demonstrate that Pisces-SA interbodies with alternative anchor fixation stabilize spinal segments equally to market standard ALIF interbodies with traditional screw fixation. As a result, Osseus has been granted the first standalone indication for an integrated ALIF device utilizing alternative fixation without the need for additional supplemental fixation.
Regarding this novel standalone indication, Dr. Thomas Jones, III, an orthopedic surgeon with The Spine Institute of Southeast Texas, said, “The ability to use this product in a truly standalone fashion, without supplemental fixation, is a game changer for my practice and patients. I now have the option to use this product in the safest and most efficient manner by limiting the procedure to an anterior-only approach. This allows for greatly reduced procedure time which is something I am constantly searching for.”
Kelly Shelton, President of Osseus, summed up the company’s excitement with the following statement:
“In an industry with many similar devices, Osseus uses cutting-edge technology and improved design techniques to innovate its implants. As a result, we are excited to announce the FDA has approved our latest portfolio addition, the Pisces-SA, as a true stand-alone ALIF device whether using traditional or alternative fixation. Not requiring supplemental fixation and additional procedures, gives surgeons new flexibility – while preserving the indication as “on label” use.
We are thrilled to offer this one-of-a-kind product to the market and are confident that giving surgeons this new option will lead to improved patient outcomes.”
For more information or to schedule a demo, email sales@osseus.com or contact Kelly Shelton, President, at kshelton@osseus.com or by calling (303) 880-0694.
About Osseus:
Osseus is a privately held, organically funded medical device firm focused on offering minimally invasive solutions to the growing US spine market. Founded in 2014 and grown in Dallas, Texas, Osseus pushes the evolution of spinal fusion products with its teams of forward-thinking surgeons, bright-minded engineers, and visionary product managers. The Osseus product line includes 11 FDA approved spinal fusion and fixation systems including: the Pisces-SA Standalone ALIF Interbody System, Black Diamond Open and Minimally Invasive Pedicle Screw Systems, White Pearl Anterior Cervical Plate, Gemini-C Cervical Interbody, Aries-TS, -TC, -L, and -A Lumbar Interbody Systems, Blue Topaz Sacroiliac Screw System, and Black Diamond POCT Posterior Cervical Screw System.