DUBLIN, March 7, 2023 /PRNewswire/ — X-Bolt, an emerging innovator of orthopedic devices, is pleased to announce that its hip fracture solution, the Pro-X1 Trochanteric Nail, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). Pro-X1 will be X-Bolt’s flagship launch in the United States.
“We are thrilled to receive FDA clearance for the Pro-X1 Trochanteric Nail,” said Brian Thornes, X-Bolt’s founder and inventor of the globally recognized product, TightRope®. “Pro-X1 builds off of the foundational expanding bolt design, clinically proven with a 0.8% cut-out rate in the largest ever hip fracture randomized controlled trial.1 We are proud to offer this innovative device to orthopedic trauma patients and are confident that it will make a significant impact in the lives of those in need.”
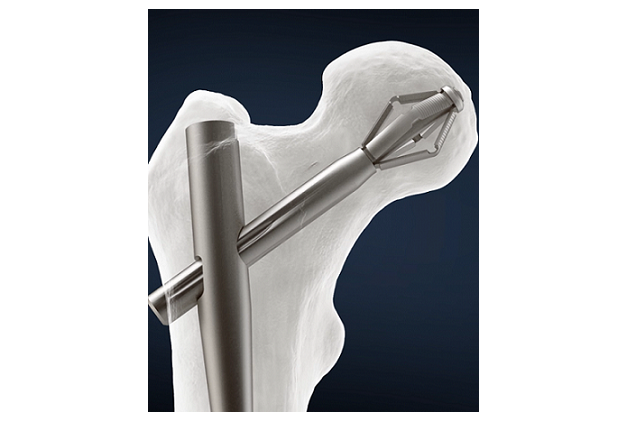
The Pro-X1 Trochanteric Nail features a titanium nail construct with a reversibly expanding bolt instead of a typical lag screw. The expanding bolt enables stronger anchorage and rotational stability of the femoral head. The system’s innovative instrumentation includes the patented Metro Jig, a curved jig with flexi-drive coupling, to streamline the operative technique.
“We are committed to developing orthopedic devices at the intersection of the best applications of our patented expanding bolt technology and the most demanding needs in orthopedic trauma,” said Thornes.
X-Bolt is actively seeking distribution partners and preparing its commercial launch ahead of the Orthopaedic Trauma Association (OTA) meeting in October 2023.
Indications for Use:
The Pro-X1™ Trochanteric Nailing System is intended for use in fracture fixation in the femur in adults with osteopenia or osteoporosis. The Pro-X1™ Trochanteric Nailing System is indicated for use in:
- Intertrochanteric and subtrochanteric fractures
- Segmental fractures
- Comminuted fractures
- Pathological fractures
- Fractures with bone loss
- Pseudoarthrosis, non-union, mal-union, and delayed union
- Surgically created defects such as osteotomies
About X-Bolt
X-Bolt is an emerging innovator of orthopedic devices that combines its patented expanding bolt technology with the most demanding needs in orthopedic trauma. The company is dedicated to improving patient outcomes and providing innovative solutions to complex surgical challenges.
For more information, visit the X-Bolt website here
1 Griffin et. al. Effect on health-related quality of life of the X-Bolt dynamic plating system versus the sliding hip screw for the fixation of trochanteric fractures of the hip in adults: the WHiTE Four randomized clinical trial. Bone Joint J. 2021.
Chief Operating Officer – Philip Kemp
philip.kemp@x-bolt.com
+353 89 616 4135
SOURCE X-Bolt Orthopedics