Company plans to begin 30-patient trial later this year
April 11, 2023
BURLINGTON, Mass.–(BUSINESS WIRE)–Bone Biologics Corporation (NASDAQ: BBLG), a developer of orthobiologic products for spine fusion markets, announces that the Human Research Ethics Committee (HREC) has approved Monash Health as the first of a planned multicenter pilot clinical trial to evaluate the Company’s NB1 bone graft in Australia.
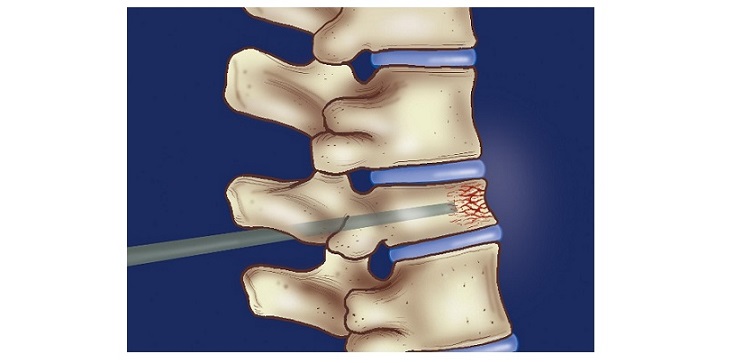
This pilot study will evaluate the safety and effectiveness of NB1 in 30 adult subjects who undergo transforaminal lumbar interbody fusion (TLIF) to treat degenerative disc disease (DDD). Inclusion criteria include patients with DDD at one level from L2-S1 who may also have up to Grade 1 spondylolisthesis or Grade 1 retrolisthesis at the involved level. The study design was previously reviewed by the U.S. Food and Drug Administration’s Division of Orthopedic Devices in a Pre-submission and is intended to support progression to a pivotal clinical study in the United States.
“We are delighted to announce this important step toward beginning our pilot study in humans and look forward to demonstrating the ability of NB1 to support the same or better fusion success rates in hard healers that was generated in our animal studies,” said Jeffrey Frelick, Bone Biologics’ president and chief executive officer.
Lumbar DDD is one of the most common causes of low back pain. DDD also leads to substantial disability, with many patients suffering from decreased ability to walk, sit, stand and/or sleep. For some people, DDD is part of the natural process of growing older and is a significant medical issue that is increasing as the global population ages.
“We believe NB1 is poised to address the problems with existing bone growth products by providing rapid, controlled and guided bone growth only in the presence of existing bone, not elsewhere in the body. Our longer-term goal is to capture a meaningful portion of the $3 billion annual global market for spine fusion products,” added Mr. Frelick.
Bone Biologics previously announced the engagement of Avania as the contract research organization for this pilot clinical trial with NB1.
About NB1
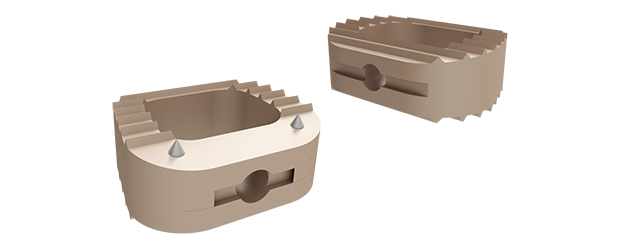
NELL-1 is a recombinant human protein Bone Biologics licensed through a technology transfer agreement with the UCLA Technology Development Group for worldwide applications. NELL-1 combined with demineralized bone matrix (DBM) forms the product candidate NB1. Bone Biologics has entered into an agreement with MTF Biologics for supplying DBM as a carrier in NB1. NELL-1 has unique properties that suggest it will be ideal in treating spinal fusion, trauma, osteoporosis and other bone-related indications, and may be especially useful among “hard healers.” This potential lies in its ability to provide rapid, specific and guided control over bone regeneration.
For the NB1 bone graft device, the inclusion of rhNELL-1 provides an ancillary osteopromotive effect that is expected to increase both the quantity and maturity of bone and to increase the rate of spinal fusion. The proposed mechanism of action for rhNELL-1 to improve bone formation is based on published research and involves classic receptor binding and intracellular signaling transduction to the nucleus to promote osteogenic gene expression and bone formation.
There is a large and established opportunity for NB1 with an estimated global market of $3 billion annually for bone graft substitutes in spine fusion for products such as growth factors, DBM, synthetic materials, stem cells and allografts. Additional, longer-term market opportunities include the $11 billion annual market for treating osteoporosis and the $8 billion annual market for treating trauma patients.
About Bone Biologics
Bone Biologics was founded to pursue regenerative medicine for bone. The Company is undertaking work with select strategic partners that builds on the preclinical research of the Nell-1 protein. Bone Biologics is currently focusing its development efforts for its bone graft substitute product on bone regeneration in spinal fusion procedures, while additionally having rights to trauma and osteoporosis applications. For more information, please visit www.bonebiologics.com.
Forward-looking Statements
Certain statements contained in this press release, including, without limitation, statements containing the words ‘’believes,” “anticipates,” “expects” and words of similar import, constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve both known and unknown risks and uncertainties. The Company’s actual results may differ materially from those anticipated in its forward-looking statements as a result of a number of factors, including those including the Company’s ability to develop our lead product NB1 and other proposed products, its ability to obtain patent protection for its technology, its ability to obtain the necessary financing to develop products and conduct the necessary clinical testing, its ability to obtain Federal Food and Drug Administration approval to market any product it may develop in the United States and to obtain any other regulatory approval necessary to market any product in other countries, its ability to market any product it may develop, its ability to create, sustain, manage or forecast its growth; its ability to attract and retain key personnel; changes in the Company’s business strategy or development plans; competition; business disruptions; adverse publicity and international, national and local general economic and market conditions and risks generally associated with an undercapitalized developing company, as well as the risks contained under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the Company’s Form S-1, Form 10-K for the year ended December 31, 2022 and the Company’s other filings with the Securities and Exchange Commission. Except as required by applicable law, we undertake no obligation to revise or update any forward-looking statements to reflect any event or circumstance that may arise after the date hereof.
Contacts
LHA Investor Relations
Kim Sutton Golodetz
212-838-3777
kgolodetz@lhai.com