May 2, 2023 – Schiedam, the Netherlands /OrthoSpineNews/ –
Cresco Spine, a new player in the field of surgical treatments of scoliosis, today announced that it has received the FDA Breakthrough Device designation for its innovative Spring Distraction System (SDSTM). This status recognizes the significant potential of the SDSTM spinal system to provide a more effective and less burdensome treatment option for young patients suffering from early onset scoliosis.
Early onset scoliosis is a severe and life-threatening condition in a vulnerable, young patient group. These patients are severely affected by this condition that may lead to deficient cardio-pulmonary development. Treatment is associated with high complication rates and usually requires multiple surgeries or other clinical interventions throughout the early years of life. Moreover, multiple hospital visits for monitoring are required, which further increases the disease burden for these young children and their parents, and ultimately leading to high healthcare costs.
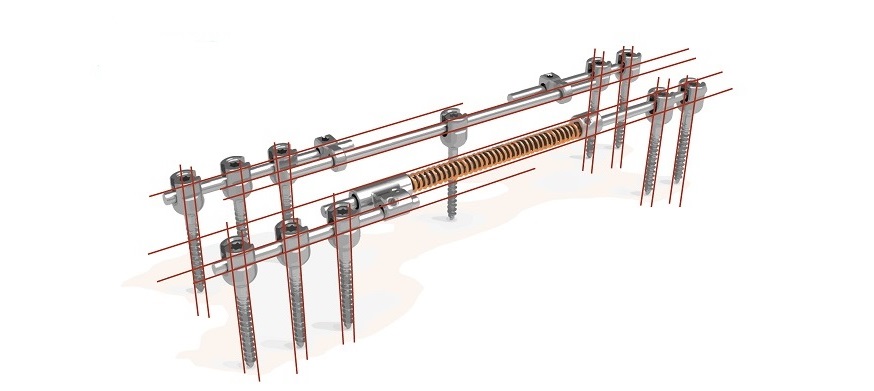
SDSTM (a proprietary spring based dynamic distraction system that works with standard of care posterior pedicle screw fixation) diminishes the number of operations and abstains from clinical lengthening interventions, while allowing three-dimensional guided spinal growth even in the most extreme scoliotic deformations. This will improve outcomes, whilst minimizing re-operations and surgical and anesthetic burden in this vulnerable patient group, besides being more costeffective.
“Receiving the FDA Breakthrough Device designation is a significant milestone for Cresco Spine,” said Dennis Magermans, CEO of Cresco Spine “This recognition underscores the potential of our innovative spinal implant system to make a real difference in the lives of patients suffering from this debilitating spinal condition. We are excited to continue working with the FDA to bring this important medical technology to market as quickly and safely as possible.”
Prof. Dr. René Castelein, orthopedic surgeon and Cresco Spine’s Chief Medical Officer comments: “SDSTM is currently successfully being tested in a significant number of patients in clinical exemption trials. We are committed to delivering innovative medical solutions in the field surgical scoliosis treatment, that improve patient outcomes and quality of life, while reducing medical costs”.
The FDA’s Breakthrough Device program is designed to expedite the development and review of innovative medical devices that have the potential to provide significant benefits over existing treatments or technologies. The program provides manufacturers with enhanced support and guidance from the FDA throughout the development process, including expedited review and priority review.
For more information about Cresco Spine and the SDSTM implant system, please visit the company’s website at www.crescospine.com
Background statements
About Cresco Spine
Cresco Spine is a medical technology company developing surgical spinal solutions focusing on improving scoliosis care. Cresco Spine was founded in 2021 as a spin-out from the University Medical Center in Utrecht, the Netherlands.
For more information, please visit https://www.cresco-spine.com
Contact information
Dennis Magermans, CEO Cresco Spine, dennis@cresco-spine.com